Male germline control of transposable elements
- PMID: 22357546
- PMCID: PMC3364930
- DOI: 10.1095/biolreprod.111.095463
Male germline control of transposable elements
Abstract
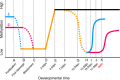
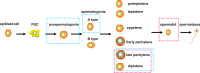
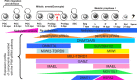
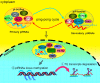
Repetitive sequences, especially transposon-derived interspersed repetitive elements, account for a large fraction of the genome in most eukaryotes. Despite the repetitive nature, these transposable elements display quantitative and qualitative differences even among species of the same lineage. Although transposable elements contribute greatly as a driving force to the biological diversity during evolution, they can induce embryonic lethality and genetic disorders as a result of insertional mutagenesis and genomic rearrangement. Temporary relaxation of the epigenetic control of retrotransposons during early germline development opens a risky window that can allow retrotransposons to escape from host constraints and to propagate abundantly in the host genome. Because germline mutations caused by retrotransposon activation are heritable and thus can be deleterious to the offspring, an adaptive strategy has evolved in host cells, especially in the germline. In this review, we will attempt to summarize general defense mechanisms deployed by the eukaryotic genome, with an emphasis on pathways utilized by the male germline to confer retrotransposon silencing.
Figures

References
-
- Biemont C, Vieira C. Genetics: junk DNA as an evolutionary force. Nature 2006; 443: 521 524 - PubMed
-
- Kidwell MG, Lisch DR. Perspective: transposable elements, parasitic DNA, and genome evolution. Evolution 2001; 55: 1 24 - PubMed
-
- Gregory TR, Hebert PD. The modulation of DNA content: proximate causes and ultimate consequences. Genome Res 1999; 9: 317 324 - PubMed
-
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J. et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002; 420: 520 562 - PubMed
-
- Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science 2004; 303: 1626 1632 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

