A practical method for patterning lumens through ECM hydrogels via viscous finger patterning
- PMID: 22357560
- PMCID: PMC3397721
- DOI: 10.1177/2211068211426694
A practical method for patterning lumens through ECM hydrogels via viscous finger patterning
Abstract
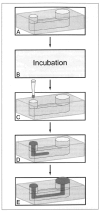
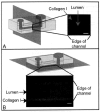
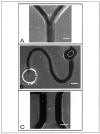
Extracellular matrix (ECM) hydrogels with patterned lumens have been used as a framework to generate more physiologically relevant models of tissues, such as vessels and mammary ducts, for biological investigations. However, these models have not found widespread use in research labs or in high-throughput screening applications in large part because the basic methods for generating the lumen structures are generally cumbersome and slow. Here we present viscous finger patterning, a technique to generate lumens through ECM hydrogels in microchannels that can be accomplished using manual or automated pipetting. Passive pumping is used to flow culture media through an unpolymerized hydrogel, creating a lumen through the hydrogel that is subsequently polymerized. Viscous finger patterning takes advantage of viscous fingering, the fluid dynamics phenomenon where a less viscous fluid will flow through and displace a more viscous fluid. We have characterized the technique and used it to create a variety of channel geometries and ECM hydrogel compositions, as well as for the generation of lumens surrounded by multiple hydrogel layers. Because viscous finger patterning can be performed with automated liquid handling systems, high-throughput generation of ECM hydrogels with patterned lumen is enabled. The ability to rapidly and cost-effectively create large numbers of lumens in natural polymers overcomes a critical barrier to the use of more physiologically relevant tissue models in a variety of biological studies and drug screening applications.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D. I. Beebe has an ownership interest in BellBrook Labs, LLC, which has licensed technology presented in this article.
Figures


Similar articles
-
A Facile and Scalable Hydrogel Patterning Method for Microfluidic 3D Cell Culture and Spheroid-in-Gel Culture Array.Biosensors (Basel). 2021 Dec 10;11(12):509. doi: 10.3390/bios11120509. Biosensors (Basel). 2021. PMID: 34940266 Free PMC article.
-
Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor.Biomaterials. 2017 Apr;123:142-154. doi: 10.1016/j.biomaterials.2017.01.037. Epub 2017 Jan 30. Biomaterials. 2017. PMID: 28167392 Free PMC article.
-
3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels.Acta Biomater. 2019 Sep 1;95:201-213. doi: 10.1016/j.actbio.2019.06.017. Epub 2019 Jun 21. Acta Biomater. 2019. PMID: 31233891 Free PMC article.
-
Biocompatibility of hydrogel-based scaffolds for tissue engineering applications.Biotechnol Adv. 2017 Sep;35(5):530-544. doi: 10.1016/j.biotechadv.2017.05.006. Epub 2017 May 27. Biotechnol Adv. 2017. PMID: 28558979 Review.
-
Extracellular matrix hydrogels from decellularized tissues: Structure and function.Acta Biomater. 2017 Feb;49:1-15. doi: 10.1016/j.actbio.2016.11.068. Epub 2016 Dec 1. Acta Biomater. 2017. PMID: 27915024 Free PMC article. Review.
Cited by
-
A Bloody Conspiracy- Blood Vessels and Immune Cells in the Tumor Microenvironment.Cancers (Basel). 2022 Sep 21;14(19):4581. doi: 10.3390/cancers14194581. Cancers (Basel). 2022. PMID: 36230504 Free PMC article. Review.
-
Review Article: Capturing the physiological complexity of the brain's neuro-vascular unit in vitro.Biomicrofluidics. 2018 Oct 16;12(5):051502. doi: 10.1063/1.5045126. eCollection 2018 Sep. Biomicrofluidics. 2018. PMID: 30364144 Free PMC article. Review.
-
Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip.PLoS One. 2016 Mar 1;11(3):e0150360. doi: 10.1371/journal.pone.0150360. eCollection 2016. PLoS One. 2016. PMID: 26930059 Free PMC article.
-
Vessel-on-a-chip models for studying microvascular physiology, transport, and function in vitro.Am J Physiol Cell Physiol. 2021 Jan 1;320(1):C92-C105. doi: 10.1152/ajpcell.00355.2020. Epub 2020 Nov 11. Am J Physiol Cell Physiol. 2021. PMID: 33176110 Free PMC article. Review.
-
Microfluidics in vascular biology research: a critical review for engineers, biologists, and clinicians.Lab Chip. 2022 Sep 27;22(19):3618-3636. doi: 10.1039/d2lc00352j. Lab Chip. 2022. PMID: 36047330 Free PMC article. Review.
References
-
- Chrobak K, Potter D, Tien J. Formation of Perfused, Functional Microvascular Tubes In Vitro. Microvasc Res. 2006;71(3):185–196. - PubMed
-
- Golden AP, Tien J. Fabrication of Microfluidic Hydrogels Using Molded Gelatin as a Sacrificial Element. Lab Chip. 2007;7(6):720–725. - PubMed
-
- Berry SM, Warren SP, Hilgart DA, Schworer AT, Pabba S, Gobin AS, Cohn RW, Keynton RS. Endothelial Cell Scaffolds Generated by 3D Direct Writing of Biodegradable Polymer Microfibers. Biomaterials. 2010;32:1–8. - PubMed
-
- Fiddes LK, Raz N, Srigunapalan S, Tumarkan E, Simmons CA, Wheeler AR, Kumacheva E. A Circular Cross-Section PDMS Microfluidics System for Replication of Cardiovascular Flow Conditions. Biomaterials. 2010;31(13):3459–3464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

