ATR cooperates with CTC1 and STN1 to maintain telomeres and genome integrity in Arabidopsis
- PMID: 22357613
- PMCID: PMC3327312
- DOI: 10.1091/mbc.E11-12-1002
ATR cooperates with CTC1 and STN1 to maintain telomeres and genome integrity in Arabidopsis
Abstract
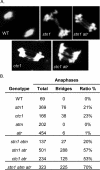
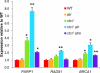
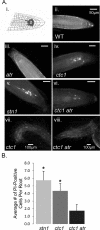
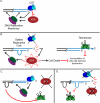
The CTC1/STN1/TEN1 (CST) complex is an essential constituent of plant and vertebrate telomeres. Here we show that CST and ATR (ataxia telangiectasia mutated [ATM] and Rad3-related) act synergistically to maintain telomere length and genome stability in Arabidopsis. Inactivation of ATR, but not ATM, temporarily rescued severe morphological phenotypes associated with ctc1 or stn1. Unexpectedly, telomere shortening accelerated in plants lacking CST and ATR. In first-generation (G1) ctc1 atr mutants, enhanced telomere attrition was modest, but in G2 ctc1 atr, telomeres shortened precipitously, and this loss coincided with a dramatic decrease in telomerase activity in G2 atr mutants. Zeocin treatment also triggered a reduction in telomerase activity, suggesting that the prolonged absence of ATR leads to a hitherto-unrecognized DNA damage response (DDR). Finally, our data indicate that ATR modulates DDR in CST mutants by limiting chromosome fusions and transcription of DNA repair genes and also by promoting programmed cell death in stem cells. We conclude that the absence of CST in Arabidopsis triggers a multifaceted ATR-dependent response to facilitate maintenance of critically shortened telomeres and eliminate cells with severe telomere dysfunction.
Figures



Similar articles
-
Arabidopsis ATM and ATR kinases prevent propagation of genome damage caused by telomere dysfunction.Plant Cell. 2011 Dec;23(12):4254-65. doi: 10.1105/tpc.111.092387. Epub 2011 Dec 9. Plant Cell. 2011. PMID: 22158468 Free PMC article.
-
POT1a and components of CST engage telomerase and regulate its activity in Arabidopsis.PLoS Genet. 2014 Oct 16;10(10):e1004738. doi: 10.1371/journal.pgen.1004738. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25329641 Free PMC article.
-
MERISTEM DISORGANIZATION1 encodes TEN1, an essential telomere protein that modulates telomerase processivity in Arabidopsis.Plant Cell. 2013 Apr;25(4):1343-54. doi: 10.1105/tpc.112.107425. Epub 2013 Apr 9. Plant Cell. 2013. PMID: 23572541 Free PMC article.
-
CST in maintaining genome stability: Beyond telomeres.DNA Repair (Amst). 2021 Jun;102:103104. doi: 10.1016/j.dnarep.2021.103104. Epub 2021 Mar 22. DNA Repair (Amst). 2021. PMID: 33780718 Free PMC article. Review.
-
CST-Polα/Primase: the second telomere maintenance machine.Genes Dev. 2023 Jul 1;37(13-14):555-569. doi: 10.1101/gad.350479.123. Epub 2023 Jul 26. Genes Dev. 2023. PMID: 37495394 Free PMC article. Review.
Cited by
-
The Linker Histone GH1-HMGA1 Is Involved in Telomere Stability and DNA Damage Repair.Plant Physiol. 2018 May;177(1):311-327. doi: 10.1104/pp.17.01789. Epub 2018 Apr 5. Plant Physiol. 2018. PMID: 29622687 Free PMC article.
-
Analysis of poly(ADP-Ribose) polymerases in Arabidopsis telomere biology.PLoS One. 2014 Feb 13;9(2):e88872. doi: 10.1371/journal.pone.0088872. eCollection 2014. PLoS One. 2014. PMID: 24551184 Free PMC article.
-
tRNA ADENOSINE DEAMINASE 3 is required for telomere maintenance in Arabidopsis thaliana.Plant Cell Rep. 2020 Dec;39(12):1669-1685. doi: 10.1007/s00299-020-02594-0. Epub 2020 Sep 21. Plant Cell Rep. 2020. PMID: 32959123 Free PMC article.
-
Emerging roles of CST in maintaining genome stability and human disease.Front Biosci (Landmark Ed). 2018 Mar 1;23(8):1564-1586. doi: 10.2741/4661. Front Biosci (Landmark Ed). 2018. PMID: 29293451 Free PMC article. Review.
-
DDM1 guards against telomere truncation in Arabidopsis.Plant Cell Rep. 2018 Mar;37(3):501-513. doi: 10.1007/s00299-017-2245-6. Epub 2018 Feb 1. Plant Cell Rep. 2018. PMID: 29392401 Free PMC article.
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11:36–42.
-
- Anderson BH, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet. 2012;44:338–342. - PubMed
-
- Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

