Rnd1 and Rnd3 targeting to lipid raft is required for p190 RhoGAP activation
- PMID: 22357615
- PMCID: PMC3327318
- DOI: 10.1091/mbc.E11-11-0900
Rnd1 and Rnd3 targeting to lipid raft is required for p190 RhoGAP activation
Abstract
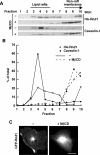
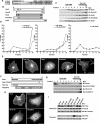
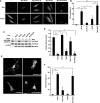
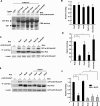
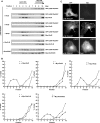
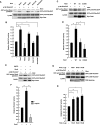
The Rnd proteins Rnd1, Rnd2, and Rnd3/RhoE are well known as key regulators of the actin cytoskeleton in various cell types, but they comprise a distinct subgroup of the Rho family in that they are GTP bound and constitutively active. Functional differences of the Rnd proteins in RhoA inhibition signaling have been reported in various cell types. Rnd1 and Rnd3 antagonize RhoA signaling by activating p190 RhoGAP, whereas Rnd2 does not. However, all the members of the Rnd family have been reported to bind directly to p190 RhoGAP and equally induce activation of p190 RhoGAP in vitro, and there is no evidence that accounts for the functional difference of the Rnd proteins in RhoA inhibition signaling. Here we report the role of the N-terminal region in signaling. Rnd1 and Rnd3, but not Rnd2, have a KERRA (Lys-Glu-Arg-Arg-Ala) sequence of amino acids in their N-terminus, which functions as the lipid raft-targeting determinant. The sequence mediates the lipid raft targeting of p190 RhoGAP correlated with its activation. Overall, our results demonstrate a novel regulatory mechanism by which differential membrane targeting governs activities of Rnd proteins to function as RhoA antagonists.
Figures
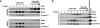


References
-
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphoring signalling. J Cell Sci. 2005;118:4689–4700. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. - PubMed
-
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

