Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly
- PMID: 22357621
- PMCID: PMC3327322
- DOI: 10.1091/mbc.E11-11-0957
Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly
Abstract
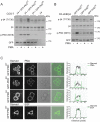
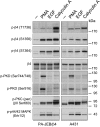
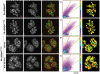
During wound healing, hemidesmome disassembly enables keratinocyte migration and proliferation. Hemidesmosome dynamics are altered downstream of epidermal growth factor (EGF) receptor activation, following the phosphorylation of integrin β4 residues S1356 and S1364, which reduces the interaction with plectin; however, this event is insufficient to drive complete hemidesmome disassembly. In the studies reported here, we used a fluorescence resonance energy transfer-based assay to demonstrate that the connecting segment and carboxy-terminal tail of the β4 cytoplasmic domain interact, which facilitates the formation of a binding platform for plectin. In addition, analysis of a β4 mutant containing a phosphomimicking aspartic acid residue at T1736 in the C-tail suggests that phosphorylation of this residue regulates the interaction with the plectin plakin domain. The aspartic acid mutation of β4 T1736 impaired hemidesmosome formation in junctional epidermolysis associated with pyloric atresia/β4 keratinocytes. Furthermore, we show that T1736 is phosphorylated downstream of protein kinase C and EGF receptor activation and is a substrate for protein kinase D1 in vitro and in cells, which requires its translocation to the plasma membrane and subsequent activation. In conclusion, we identify T1736 as a novel phosphorylation site that contributes to the regulation of hemidesmome disassembly, a dynamically regulated process involving the concerted phosphorylation of multiple β4 residues.
Figures





Similar articles
-
PKD2 and RSK1 Regulate Integrin β4 Phosphorylation at Threonine 1736.PLoS One. 2015 Nov 18;10(11):e0143357. doi: 10.1371/journal.pone.0143357. eCollection 2015. PLoS One. 2015. PMID: 26580203 Free PMC article.
-
EGF-induced MAPK signaling inhibits hemidesmosome formation through phosphorylation of the integrin {beta}4.J Biol Chem. 2010 Nov 26;285(48):37650-62. doi: 10.1074/jbc.M110.138818. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870721 Free PMC article.
-
Serine phosphorylation of the integrin beta4 subunit is necessary for epidermal growth factor receptor induced hemidesmosome disruption.Mol Biol Cell. 2007 Sep;18(9):3512-22. doi: 10.1091/mbc.e07-04-0306. Epub 2007 Jul 5. Mol Biol Cell. 2007. PMID: 17615294 Free PMC article.
-
Regulation of hemidesmosome disassembly by growth factor receptors.Curr Opin Cell Biol. 2008 Oct;20(5):589-96. doi: 10.1016/j.ceb.2008.05.001. Epub 2008 Jun 24. Curr Opin Cell Biol. 2008. PMID: 18583123 Review.
-
Current insights into the formation and breakdown of hemidesmosomes.Trends Cell Biol. 2006 Jul;16(7):376-83. doi: 10.1016/j.tcb.2006.05.004. Epub 2006 Jun 6. Trends Cell Biol. 2006. PMID: 16757171 Review.
Cited by
-
Role of Integrins in Resistance to Therapies Targeting Growth Factor Receptors in Cancer.Cancers (Basel). 2019 May 17;11(5):692. doi: 10.3390/cancers11050692. Cancers (Basel). 2019. PMID: 31109009 Free PMC article. Review.
-
Roles of Integrin α6β4 Glycosylation in Cancer.Cancers (Basel). 2017 Jul 5;9(7):79. doi: 10.3390/cancers9070079. Cancers (Basel). 2017. PMID: 28678156 Free PMC article. Review.
-
Plectin: Dual Participation in Tumor Progression.Biomolecules. 2024 Aug 24;14(9):1050. doi: 10.3390/biom14091050. Biomolecules. 2024. PMID: 39334817 Free PMC article. Review.
-
Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy.J Histochem Cytochem. 2013 Feb;61(2):101-15. doi: 10.1369/0022155412467635. Epub 2012 Oct 23. J Histochem Cytochem. 2013. PMID: 23092791 Free PMC article. Review.
-
EGFR-dependent tyrosine phosphorylation of integrin β4 is not required for downstream signaling events in cancer cell lines.Sci Rep. 2021 Apr 21;11(1):8675. doi: 10.1038/s41598-021-88134-6. Sci Rep. 2021. PMID: 33883672 Free PMC article.
References
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. - PubMed
-
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. - PubMed
-
- Garcia-Alvarez B, Bobkov A, Sonnenberg A, de Pereda JM. Structural and functional analysis of the actin binding domain of plectin suggests alternative mechanisms for binding to F-actin and integrin beta4. Structure. 2003;11:615–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

