Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly
- PMID: 22357621
- PMCID: PMC3327322
- DOI: 10.1091/mbc.E11-11-0957
Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly
Abstract
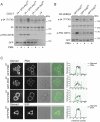
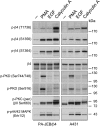
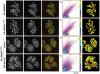
During wound healing, hemidesmome disassembly enables keratinocyte migration and proliferation. Hemidesmosome dynamics are altered downstream of epidermal growth factor (EGF) receptor activation, following the phosphorylation of integrin β4 residues S1356 and S1364, which reduces the interaction with plectin; however, this event is insufficient to drive complete hemidesmome disassembly. In the studies reported here, we used a fluorescence resonance energy transfer-based assay to demonstrate that the connecting segment and carboxy-terminal tail of the β4 cytoplasmic domain interact, which facilitates the formation of a binding platform for plectin. In addition, analysis of a β4 mutant containing a phosphomimicking aspartic acid residue at T1736 in the C-tail suggests that phosphorylation of this residue regulates the interaction with the plectin plakin domain. The aspartic acid mutation of β4 T1736 impaired hemidesmosome formation in junctional epidermolysis associated with pyloric atresia/β4 keratinocytes. Furthermore, we show that T1736 is phosphorylated downstream of protein kinase C and EGF receptor activation and is a substrate for protein kinase D1 in vitro and in cells, which requires its translocation to the plasma membrane and subsequent activation. In conclusion, we identify T1736 as a novel phosphorylation site that contributes to the regulation of hemidesmome disassembly, a dynamically regulated process involving the concerted phosphorylation of multiple β4 residues.
Figures





References
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. - PubMed
-
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. - PubMed
-
- Garcia-Alvarez B, Bobkov A, Sonnenberg A, de Pereda JM. Structural and functional analysis of the actin binding domain of plectin suggests alternative mechanisms for binding to F-actin and integrin beta4. Structure. 2003;11:615–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

