Attenuation of TGF-β signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells
- PMID: 22357622
- PMCID: PMC3327327
- DOI: 10.1091/mbc.E11-10-0849
Attenuation of TGF-β signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells
Abstract
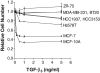
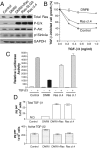
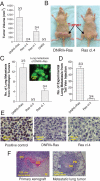
The molecular mechanisms that drive triple-negative, basal-like breast cancer progression are elusive. Few molecular targets have been identified for the prevention or treatment of this disease. Here we developed a series of isogenic basal-like human mammary epithelial cells (HMECs) with altered transforming growth factor-β (TGF-β) sensitivity and different malignancy, resembling a full spectrum of basal-like breast carcinogenesis, and determined the molecular mechanisms that contribute to oncogene-induced transformation of basal-like HMECs when TGF-β signaling is attenuated. We found that expression of a dominant-negative type II receptor (DNRII) of TGF-β abrogated autocrine TGF-β signaling in telomerase-immortalized HMECs and suppressed H-Ras-V12-induced senescence-like growth arrest (SLGA). Furthermore, coexpression of DNRII and H-Ras-V12 rendered HMECs highly tumorigenic and metastatic in vivo in comparison with H-Ras-V12-transformed HMECs that spontaneously escaped H-Ras-V12-induced SLGA. Microarray analysis revealed that p21 was the major player mediating Ras-induced SLGA, and attenuated or loss of p21 expression contributed to the escape from SLGA when autocrine TGF-β signaling was blocked in HMECs. Furthermore, knockdown of p21 also suppressed H-Ras-V12-induced SLGA. Our results identify that autocrine TGF-β signaling is an integral part of the cellular anti-transformation network by suppressing the expression of a host of genes, including p21-regulated genes, that mediate oncogene-induced transformation in basal-like breast cancer.
Figures



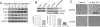
References
-
- Adorno M, et al. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. - PubMed
-
- Bandyopadhyay A, Zhu Y, Cibull ML, Bao L, Chen C, Sun L. A soluble transforming growth factor beta type III receptor suppresses tumorigenicity and metastasis of human breast cancer MDA-MB-231 cells. Cancer Res. 1999;59:5041–5046. - PubMed
-
- Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
-
- Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. - PubMed
-
- Bild AH, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

