Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan
- PMID: 22357630
- PMCID: PMC3311780
- DOI: 10.4049/jimmunol.1102863
Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan
Abstract
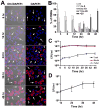
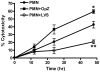
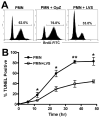
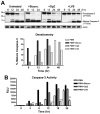
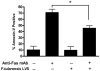
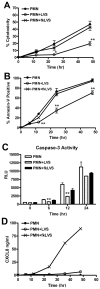
Francisella tularensis is a facultative intracellular bacterium that infects many cell types, including neutrophils. We demonstrated previously that F. tularensis inhibits NADPH oxidase assembly and activity and then escapes the phagosome to the cytosol, but effects on other aspects of neutrophil function are unknown. Neutrophils are short-lived cells that undergo constitutive apoptosis, and phagocytosis typically accelerates this process. We now demonstrate that F. tularensis significantly inhibited neutrophil apoptosis as indicated by morphologic analysis as well as annexin V and TUNEL staining. Thus, ∼80% of infected neutrophils remained viable at 48 h compared with ∼50% of control cells, and ∼40% of neutrophils that ingested opsonized zymosan. In keeping with this finding, processing and activation of procaspases-8, -9, and -3 were markedly diminished and delayed. F. tularensis also significantly impaired apoptosis triggered by Fas crosslinking. Of note, these effects were dose dependent and could be conferred by either intracellular or extracellular live bacteria, but not by formalin-killed organisms or isolated LPS and capsule, and were not affected by disruption of wbtA2 or FTT1236/FTL0708-genes required for LPS O-antigen and capsule biosynthesis. In summary, we demonstrate that F. tularensis profoundly impairs constitutive neutrophil apoptosis via effects on the intrinsic and extrinsic pathways, and thereby define a new aspect of innate immune evasion by this organism. As defects in neutrophil turnover prevent resolution of inflammation, our findings also suggest a mechanism that may in part account for the neutrophil accumulation, granuloma formation, and severe tissue damage that characterizes lethal pneumonic tularemia.
Conflict of interest statement
Figures
References
-
- Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. - PubMed
-
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. - PubMed
-
- Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–3992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

