Lapatinib and obatoclax kill tumor cells through blockade of ERBB1/3/4 and through inhibition of BCL-XL and MCL-1
- PMID: 22357666
- PMCID: PMC3336802
- DOI: 10.1124/mol.112.077586
Lapatinib and obatoclax kill tumor cells through blockade of ERBB1/3/4 and through inhibition of BCL-XL and MCL-1
Abstract
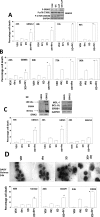
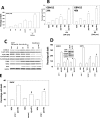
Prior studies in breast cancer cells have shown that lapatinib and obatoclax interact in a greater than additive fashion to cause cell death and do so through a toxic form of autophagy. The present studies sought to extend our analyses to the central nervous system (CNS) tumor cells and to further define mechanisms of drug action. Lapatinib and obatoclax killed multiple CNS tumor isolates. Cells lacking PTEN (phosphatase and tensin homolog on chromosome 10) function were relatively resistant to drug combination lethality; expression of PTEN in PTEN-null cells restored drug sensitivity, and knockdown of PTEN promoted drug resistance. On the basis of knockdown of ERBB1-4 (erythroblastic leukemia viral oncogene homolog 1-4), we discovered that the inhibition of ERBB1/3/4 receptors were most important for enhancing obatoclax lethality rather than ERBB2. In parallel, we noted in CNS tumor cells that knockdown of BCL-xL (B-cell lymphoma-extra large)and MCL-1 (myeloid cell leukemia-1) interacted in an additive fashion to facilitate lapatinib lethality. Pretreatment of tumor cells with obatoclax enhanced the lethality of lapatinib to a greater extent than concomitant treatment. Treatment of animals carrying orthotopic CNS tumor isolates with lapatinib- and obatoclax-prolonged survival. Altogether, our data show that lapatinib and obatoclax therapy could be of use in the treatment of tumors located in the CNS.
Figures




Similar articles
-
Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo.Cancer Biol Ther. 2010 Nov 1;10(9):903-17. doi: 10.4161/cbt.10.9.13273. Epub 2010 Nov 1. Cancer Biol Ther. 2010. PMID: 20855960 Free PMC article.
-
Obatoclax and lapatinib interact to induce toxic autophagy through NOXA.Mol Pharmacol. 2012 Apr;81(4):527-40. doi: 10.1124/mol.111.076851. Epub 2012 Jan 4. Mol Pharmacol. 2012. PMID: 22219388 Free PMC article.
-
Lapatinib and obatoclax kill breast cancer cells through reactive oxygen species-dependent endoplasmic reticulum stress.Mol Pharmacol. 2012 Dec;82(6):1217-29. doi: 10.1124/mol.112.081539. Epub 2012 Sep 18. Mol Pharmacol. 2012. PMID: 22989520 Free PMC article.
-
Sorafenib/regorafenib and lapatinib interact to kill CNS tumor cells.J Cell Physiol. 2015 Jan;230(1):131-9. doi: 10.1002/jcp.24689. J Cell Physiol. 2015. PMID: 24911215 Free PMC article.
-
Lapatinib in breast cancer - the predictive significance of HER1 (EGFR), HER2, PTEN and PIK3CA genes and lapatinib plasma level assessment.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010 Dec;154(4):281-8. doi: 10.5507/bp.2010.043. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010. PMID: 21293538 Review.
Cited by
-
The role of tumour-stromal interactions in modifying drug response: challenges and opportunities.Nat Rev Drug Discov. 2013 Mar;12(3):217-28. doi: 10.1038/nrd3870. Nat Rev Drug Discov. 2013. PMID: 23449307 Review.
-
Histone deacetylase inhibitors restore toxic BH3 domain protein expression in anoikis-resistant mammary and brain cancer stem cells, thereby enhancing the response to anti-ERBB1/ERBB2 therapy.Cancer Biol Ther. 2013 Oct 1;14(10):982-96. doi: 10.4161/cbt.26234. Epub 2013 Aug 22. Cancer Biol Ther. 2013. PMID: 24025251 Free PMC article.
-
Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR- ErbB2 signaling with lapatinib.Mol Oncol. 2013 Jun;7(3):497-512. doi: 10.1016/j.molonc.2013.01.001. Epub 2013 Jan 11. Mol Oncol. 2013. PMID: 23375777 Free PMC article.
-
Regulation of Apoptosis by HER2 in Breast Cancer.J Carcinog Mutagen. 2013;2013(Suppl 7):003. doi: 10.4172/2157-2518.S7-003. Epub 2013 Jun 26. J Carcinog Mutagen. 2013. PMID: 27088047 Free PMC article.
-
A Holistic Review on the Current and Future Status of Biology-Driven and Broad-Spectrum Therapeutic Options for Medulloblastoma.Cureus. 2022 Mar 24;14(3):e23447. doi: 10.7759/cureus.23447. eCollection 2022 Mar. Cureus. 2022. PMID: 35481313 Free PMC article. Review.
References
-
- Alva AS, Gultekin SH, Baehrecke EH. (2004) Autophagy in human tumors: cell survival or death? Cell Death Differ 11:1046–1048 - PubMed
-
- Awada A, Saliba W, Bozovic-Spasojevic I. (2011) Lapatinib ditosylate: expanding therapeutic options for receptor tyrosine-protein kinase erbB-2-positive breast cancer. Drugs Today (Barc) 47:335–345 - PubMed
-
- Berezowska S, Schlegel J. (2011) Targeting ErbB receptors in high-grade glioma. Curr Pharm Des 17:2468–2487 - PubMed
-
- Bigner SH, Vogelstein B. (1990) Cytogenetics and molecular genetics of malignant gliomas and medulloblastoma. Brain Pathol 1:12–18 - PubMed
-
- Depowski PL, Rosenthal SI, Ross JS. (2001) Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol 14:672–676 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

