Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]
- PMID: 22357757
- PMCID: PMC3309714
- DOI: 10.1073/pnas.1117963109
Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]
Erratum in
- Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5905
Abstract
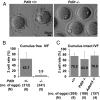
A disintegrin and metalloproteinase 3 (ADAM3) is a sperm membrane protein critical for both sperm migration from the uterus into the oviduct and sperm primary binding to the zona pellucida (ZP). Here we show that the testis-specific protein disulfide isomerase homolog (PDILT) cooperates with the testis-specific calreticulin-like chaperone, calsperin (CALR3), in the endoplasmic reticulum and plays an indispensable role in the disulfide-bond formation and folding of ADAM3. Pdilt(-/-) mice were male infertile because ADAM3 could not be folded properly and transported to the sperm surface without the PDILT/CALR3 complex. Peculiarly we find that not only Pdilt(-/-), but also Adam3(-/-), spermatozoa effectively fertilize eggs when the eggs are surrounded in cumulus oophorus. These findings reveal that ADAM3 requires testis-specific private chaperones to be folded properly and that the principle role of ADAM3 is for sperm migration into the oviduct but not for the fertilization event. Moreover, the importance of primary sperm ZP binding, which has been thought to be a critical step in mammalian fertilization, should be reconsidered.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Calsperin is a testis-specific chaperone required for sperm fertility.J Biol Chem. 2011 Feb 18;286(7):5639-46. doi: 10.1074/jbc.M110.140152. Epub 2010 Dec 3. J Biol Chem. 2011. PMID: 21131354 Free PMC article.
-
Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa.Mol Biol Cell. 2012 Jul;23(14):2671-9. doi: 10.1091/mbc.E11-12-1025. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621904 Free PMC article.
-
Dissecting the PRSS37 interactome and potential mechanisms leading to ADAM3 loss in PRSS37-null sperm.J Cell Sci. 2021 May 15;134(10):jcs258426. doi: 10.1242/jcs.258426. Epub 2021 May 24. J Cell Sci. 2021. PMID: 34028541
-
Sperm-egg interaction and gene manipulated animals.Soc Reprod Fertil Suppl. 2007;65:363-71. Soc Reprod Fertil Suppl. 2007. PMID: 17644977 Review.
-
Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals.Cells. 2021 Jan 12;10(1):133. doi: 10.3390/cells10010133. Cells. 2021. PMID: 33445482 Free PMC article. Review.
Cited by
-
Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models.Sci Rep. 2016 Sep 15;6:33589. doi: 10.1038/srep33589. Sci Rep. 2016. PMID: 27627854 Free PMC article.
-
An Emerging Role of TEX101 Protein as a Male Infertility Biomarker.EJIFCC. 2014 Apr 28;25(1):9-26. eCollection 2014 Apr. EJIFCC. 2014. PMID: 27683454 Free PMC article.
-
1700029I15Rik orchestrates the biosynthesis of acrosomal membrane proteins required for sperm-egg interaction.Proc Natl Acad Sci U S A. 2023 Feb 21;120(8):e2207263120. doi: 10.1073/pnas.2207263120. Epub 2023 Feb 14. Proc Natl Acad Sci U S A. 2023. PMID: 36787362 Free PMC article.
-
MORN2 regulates the morphology and energy metabolism of mitochondria and is required for male fertility in mice.J Transl Med. 2024 Mar 5;22(1):240. doi: 10.1186/s12967-024-05010-3. J Transl Med. 2024. PMID: 38443933 Free PMC article.
-
Mechanical tuning of mammalian sperm behaviour by hyperactivation, rheology and substrate adhesion: a numerical exploration.J R Soc Interface. 2016 Nov;13(124):20160633. doi: 10.1098/rsif.2016.0633. J R Soc Interface. 2016. PMID: 27852807 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

