Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons
- PMID: 22357797
- PMCID: PMC3362278
- DOI: 10.1152/jn.00528.2011
Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons
Abstract
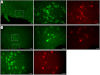
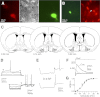
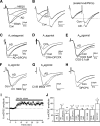
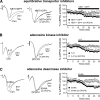
Adenosine has been proposed as an endogenous homeostatic sleep factor that accumulates during waking and inhibits wake-active neurons to promote sleep. It has been specifically hypothesized that adenosine decreases wakefulness and promotes sleep recovery by directly inhibiting wake-active neurons of the basal forebrain (BF), particularly BF cholinergic neurons. We previously showed that adenosine directly inhibits BF cholinergic neurons. Here, we investigated 1) how adenosine modulates glutamatergic input to BF cholinergic neurons and 2) how adenosine uptake and adenosine metabolism are involved in regulating extracellular levels of adenosine. Our experiments were conducted using whole cell patch-clamp recordings in mouse brain slices. We found that in BF cholinergic neurons, adenosine reduced the amplitude of AMPA-mediated evoked glutamatergic excitatory postsynaptic currents (EPSCs) and decreased the frequency of spontaneous and miniature EPSCs through presynaptic A(1) receptors. Thus we have demonstrated that in addition to directly inhibiting BF cholinergic neurons, adenosine depresses excitatory inputs to these neurons. It is therefore possible that both direct and indirect inhibition may synergistically contribute to the sleep-promoting effects of adenosine in the BF. We also found that blocking the influx of adenosine through the equilibrative nucleoside transporters or inhibiting adenosine kinase and adenosine deaminase increased endogenous adenosine inhibitory tone, suggesting a possible mechanism through which adenosine extracellular levels in the basal forebrain are regulated.
Figures

References
-
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12: 435–440, 2001 - PubMed
-
- Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci 8: s683–s693, 2003 - PubMed
-
- Allen Institute for Brain Science. Allen Brain Atlas. (Online). http://www.brain-map.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

