Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits
- PMID: 22357798
- PMCID: PMC3362282
- DOI: 10.1152/jn.01129.2011
Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits
Abstract
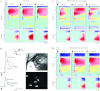
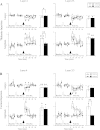
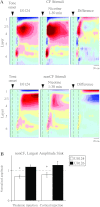
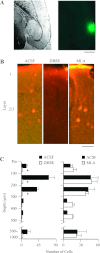
Activation of nicotinic acetylcholine receptors (nAChRs) by systemic nicotine enhances sensory-cognitive function and sensory-evoked cortical responses. Although nAChRs mediate fast neurotransmission at many synapses in the nervous system, nicotinic regulation of cortical processing is neuromodulatory. To explore potential mechanisms of nicotinic neuromodulation, we examined whether intracellular signal transduction involving mitogen-activated protein kinase (MAPK) contributes to regulation of tone-evoked responses in primary auditory cortex (A1) in the mouse. Systemic nicotine enhanced characteristic frequency (CF) tone-evoked current-source density (CSD) profiles in A1, including the shortest-latency (presumed thalamocortical) current sink in layer 4 and longer-latency (presumed intracortical) sinks in layers 2-4, by increasing response amplitudes and decreasing response latencies. Microinjection of the MAPK kinase (MEK) inhibitor U0126 into the thalamus, targeting the auditory thalamocortical pathway, blocked the effect of nicotine on the initial (thalamocortical) CSD component but did not block enhancement of longer-latency (intracortical) responses. Conversely, microinjection of U0126 into supragranular layers of A1 blocked nicotine's effect on intracortical, but not thalamocortical, CSD components. Simultaneously with enhancement of CF-evoked responses, responses to spectrally distant (nonCF) stimuli were reduced, implying nicotinic "sharpening" of frequency receptive fields, an effect also blocked by MEK inhibition. Consistent with these physiological results, acoustic stimulation with nicotine produced immunolabel for activated MAPK in A1, primarily in layer 2/3 cell bodies. Immunolabel was blocked by intracortical microinjection of the nAChR antagonist dihydro-β-erythroidine, but not methyllycaconitine, implicating α4β2*, but not α7, nAChRs. Thus activation of MAPK in functionally distinct forebrain circuits--thalamocortical, local intracortical, and long-range intracortical--underlies nicotinic neuromodulation of A1.
Figures


Similar articles
-
Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions.eNeuro. 2017 Jun 22;4(3):ENEURO.0192-17.2017. doi: 10.1523/ENEURO.0192-17.2017. eCollection 2017 May-Jun. eNeuro. 2017. PMID: 28660244 Free PMC article.
-
Heightened nicotinic regulation of auditory cortex during adolescence.J Neurosci. 2011 Oct 5;31(40):14367-77. doi: 10.1523/JNEUROSCI.1705-11.2011. J Neurosci. 2011. PMID: 21976522 Free PMC article.
-
Alpha-2 nicotinic acetylcholine receptors regulate spectral integration in auditory cortex.Front Neural Circuits. 2024 Nov 1;18:1492452. doi: 10.3389/fncir.2024.1492452. eCollection 2024. Front Neural Circuits. 2024. PMID: 39553292 Free PMC article.
-
Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing.Neuropharmacology. 2009;56 Suppl 1(Suppl 1):254-62. doi: 10.1016/j.neuropharm.2008.07.020. Epub 2008 Jul 22. Neuropharmacology. 2009. PMID: 18692078 Free PMC article. Review.
-
Functional connectivity and cholinergic modulation in auditory cortex.Neurosci Biobehav Rev. 2011 Nov;35(10):2058-63. doi: 10.1016/j.neubiorev.2010.11.010. Epub 2010 Dec 7. Neurosci Biobehav Rev. 2011. PMID: 21144860 Free PMC article. Review.
Cited by
-
Nicotine enhances auditory processing in healthy and normal-hearing young adult nonsmokers.Psychopharmacology (Berl). 2020 Mar;237(3):833-840. doi: 10.1007/s00213-019-05421-x. Epub 2019 Dec 12. Psychopharmacology (Berl). 2020. PMID: 31832719 Free PMC article. Clinical Trial.
-
Convergence of nicotine-induced and auditory-evoked neural activity activates ERK in auditory cortex.Synapse. 2013 Aug;67(8):455-68. doi: 10.1002/syn.21647. Epub 2013 Mar 8. Synapse. 2013. PMID: 23401204 Free PMC article.
-
Effects of Locomotion in Auditory Cortex Are Not Mediated by the VIP Network.Front Neural Circuits. 2021 Apr 7;15:618881. doi: 10.3389/fncir.2021.618881. eCollection 2021. Front Neural Circuits. 2021. PMID: 33897378 Free PMC article.
-
Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity.Front Neural Circuits. 2014 Mar 11;8:17. doi: 10.3389/fncir.2014.00017. eCollection 2014. Front Neural Circuits. 2014. PMID: 24653678 Free PMC article. Review.
-
Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions.eNeuro. 2017 Jun 22;4(3):ENEURO.0192-17.2017. doi: 10.1523/ENEURO.0192-17.2017. eCollection 2017 May-Jun. eNeuro. 2017. PMID: 28660244 Free PMC article.
References
-
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res 1252: 130–142, 2009 - PubMed
-
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119: 965–977, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

