Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation
- PMID: 22357842
- PMCID: PMC3427762
- DOI: 10.1523/JNEUROSCI.5057-11.2012
Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation
Abstract
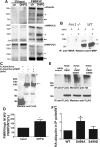
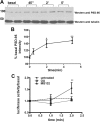
Fragile X syndrome is caused by the loss of fragile X mental retardation protein (FMRP), which represses and reversibly regulates the translation of a subset of mRNAs in dendrites. Protein synthesis can be rapidly stimulated by mGluR-induced and protein phosphatase 2a (PP2A)-mediated dephosphorylation of FMRP, which is coupled to the dissociation of FMRP and target mRNAs from miRNA-induced silencing complexes. Here, we report the rapid ubiquitination and ubiquitin proteasome system (UPS)-mediated degradation of FMRP in dendrites upon DHPG (3,5-dihydroxyphenylglycine) stimulation in cultured rat neurons. Using inhibitors to PP2A and FMRP phosphomutants, degradation of FMRP was observed to depend on its prior dephosphorylation. Translational induction of an FMRP target, postsynaptic density-95 mRNA, required both PP2A and UPS. Thus, control of FMRP levels at the synapse by dephosphorylation-induced and UPS-mediated degradation provides a mode to regulate protein synthesis.
Figures

Similar articles
-
Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression.J Neurosci. 2012 Apr 25;32(17):5924-36. doi: 10.1523/JNEUROSCI.4650-11.2012. J Neurosci. 2012. PMID: 22539853 Free PMC article.
-
FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A.J Neurosci. 2007 Dec 26;27(52):14349-57. doi: 10.1523/JNEUROSCI.2969-07.2007. J Neurosci. 2007. PMID: 18160642 Free PMC article.
-
Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling.Mol Cell. 2011 Jun 10;42(5):673-88. doi: 10.1016/j.molcel.2011.05.006. Mol Cell. 2011. PMID: 21658607 Free PMC article.
-
Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse.Sci Signal. 2008 Feb 5;1(5):pe6. doi: 10.1126/stke.15pe6. Sci Signal. 2008. PMID: 18272470 Review.
-
Aberrant RNA translation in fragile X syndrome: From FMRP mechanisms to emerging therapeutic strategies.Brain Res. 2018 Aug 15;1693(Pt A):24-36. doi: 10.1016/j.brainres.2018.04.008. Epub 2018 Apr 10. Brain Res. 2018. PMID: 29653083 Free PMC article. Review.
Cited by
-
DSCR1 interacts with FMRP and is required for spine morphogenesis and local protein synthesis.EMBO J. 2012 Sep 12;31(18):3655-66. doi: 10.1038/emboj.2012.190. Epub 2012 Aug 3. EMBO J. 2012. PMID: 22863780 Free PMC article.
-
Single-Molecule Imaging of PSD-95 mRNA Translation in Dendrites and Its Dysregulation in a Mouse Model of Fragile X Syndrome.J Neurosci. 2015 May 6;35(18):7116-30. doi: 10.1523/JNEUROSCI.2802-14.2015. J Neurosci. 2015. PMID: 25948262 Free PMC article.
-
Phosphorylated fragile X mental retardation protein at serine 499, is reduced in cerebellar vermis and superior frontal cortex of subjects with autism: implications for fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling.Mol Autism. 2013 Nov 1;4(1):41. doi: 10.1186/2040-2392-4-41. Mol Autism. 2013. PMID: 24180586 Free PMC article.
-
The Role of Dynamic miRISC During Neuronal Development.Front Mol Biosci. 2020 Jan 31;7:8. doi: 10.3389/fmolb.2020.00008. eCollection 2020. Front Mol Biosci. 2020. PMID: 32118035 Free PMC article. Review.
-
Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease.Semin Cell Dev Biol. 2018 May;77:51-62. doi: 10.1016/j.semcdb.2017.09.035. Epub 2017 Oct 14. Semin Cell Dev Biol. 2018. PMID: 28969983 Free PMC article. Review.
References
-
- Banerjee S, Neveu P, Kosik KS. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–884. - PubMed
-
- Bingol B, Schuman EM. A proteasome-sensitive connection between PSD-95 and GluR1 endocytosis. Neuropharmacology. 2004;47:755–763. - PubMed
-
- Ceman S, O'Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12:3295–3305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
