Phospholipase C-mediated suppression of dark noise enables single-photon detection in Drosophila photoreceptors
- PMID: 22357856
- PMCID: PMC3319679
- DOI: 10.1523/JNEUROSCI.5221-11.2012
Phospholipase C-mediated suppression of dark noise enables single-photon detection in Drosophila photoreceptors
Abstract
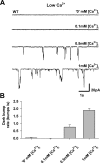
Drosophila photoreceptor cells use the ubiquitous G-protein-mediated phospholipase C (PLC) cascade to achieve ultimate single-photon sensitivity. This is manifested in the single-photon responses (quantum bumps). In photoreceptor cells, dark activation of G(q)α molecules occurs spontaneously and produces unitary dark events (dark bumps). A high rate of spontaneous G(q)α activation and dark bump production potentially hampers single-photon detection. We found that in wild-type flies the in vivo rate of spontaneous G(q)α activation is very high. Nevertheless, this high rate is not manifested in a substantially high rate of dark bumps. Therefore, it is unclear how phototransduction suppresses dark bump production arising from spontaneous G(q)α activation, while still maintaining high-fidelity representation of single photons. In this study we show that reduced PLC catalytic activity selectively suppressed production of dark bumps but not light-induced bumps. Manipulations of PLC activity using PLC mutant flies and Ca(2+) modulations revealed that a critical level of PLC activity is required to induce bump production. The required minimal level of PLC activity selectively suppressed random production of single G(q)α-activated dark bumps despite a high rate of spontaneous G(q)α activation. This minimal PLC activity level is reliably obtained by photon-induced synchronized activation of several neighboring G(q)α molecules activating several PLC molecules, but not by random activation of single G(q)α molecules. We thus demonstrate how a G-protein-mediated transduction system, with PLC as its target, selectively suppresses its intrinsic noise while preserving reliable signaling.
Figures




Similar articles
-
Functional cooperation between the IP3 receptor and phospholipase C secures the high sensitivity to light of Drosophila photoreceptors in vivo.J Neurosci. 2015 Feb 11;35(6):2530-46. doi: 10.1523/JNEUROSCI.3933-14.2015. J Neurosci. 2015. PMID: 25673847 Free PMC article.
-
Suppression of Gq and PLC gene expression has a small effect on quantum bumps in vivo in Periplaneta americana.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Jul;206(4):597-610. doi: 10.1007/s00359-020-01417-7. Epub 2020 Apr 13. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 32285147 Free PMC article.
-
Common mechanisms regulating dark noise and quantum bump amplification in Drosophila photoreceptors.J Neurophysiol. 2013 Apr;109(8):2044-55. doi: 10.1152/jn.00001.2013. Epub 2013 Jan 30. J Neurophysiol. 2013. PMID: 23365183 Free PMC article.
-
The Drosophila light-activated TRP and TRPL channels - Targets of the phosphoinositide signaling cascade.Prog Retin Eye Res. 2018 Sep;66:200-219. doi: 10.1016/j.preteyeres.2018.05.001. Epub 2018 May 5. Prog Retin Eye Res. 2018. PMID: 29738822 Review.
-
Assay for G protein-dependent activation of phospholipase C beta using purified protein components.Methods Mol Biol. 2004;237:67-75. doi: 10.1385/1-59259-430-1:67. Methods Mol Biol. 2004. PMID: 14501039 Review.
Cited by
-
Electrophysiological Method for Whole-cell Voltage Clamp Recordings from Drosophila Photoreceptors.J Vis Exp. 2017 Jun 13;(124):55627. doi: 10.3791/55627. J Vis Exp. 2017. PMID: 28654039 Free PMC article.
-
Biophotons Contribute to Retinal Dark Noise.Neurosci Bull. 2016 Jun;32(3):246-52. doi: 10.1007/s12264-016-0029-6. Epub 2016 Apr 8. Neurosci Bull. 2016. PMID: 27059222 Free PMC article.
-
Compartmentalization and Ca2+ buffering are essential for prevention of light-induced retinal degeneration.J Neurosci. 2012 Oct 17;32(42):14696-708. doi: 10.1523/JNEUROSCI.2456-12.2012. J Neurosci. 2012. PMID: 23077055 Free PMC article.
-
Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids.J Neurosci. 2015 Feb 11;35(6):2731-46. doi: 10.1523/JNEUROSCI.1150-14.2015. J Neurosci. 2015. PMID: 25673862 Free PMC article.
-
The Phosphorylation State of the Drosophila TRP Channel Modulates the Frequency Response to Oscillating Light In Vivo.J Neurosci. 2017 Apr 12;37(15):4213-4224. doi: 10.1523/JNEUROSCI.3670-16.2017. Epub 2017 Mar 17. J Neurosci. 2017. PMID: 28314815 Free PMC article.
References
-
- Barash S, Minke B. Is the receptor potential of fly photoreceptors a summation of single-photon responses? Comments Theoretical Biology. 1994;3:229–263.
-
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. - PubMed
-
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. - PubMed
-
- Cook B, Bar-Yaacov M, Cohen Ben-Ami H, Goldstein RE, Paroush Z, Selinger Z, Minke B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol. 2000;2:296–301. - PubMed
-
- Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J Neurophysiol. 2009;101:2372–2379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
