Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions
- PMID: 22357857
- PMCID: PMC3548241
- DOI: 10.1523/JNEUROSCI.5780-11.2012
Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions
Abstract
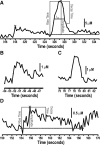
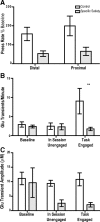
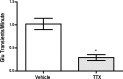
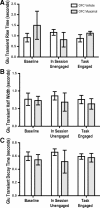
The ability to make rapid, informed decisions about whether or not to engage in a sequence of actions to earn reward is essential for survival. Modeling in rodents has demonstrated a critical role for the basolateral amygdala (BLA) in such reward-seeking actions, but the precise neurochemical underpinnings are not well understood. Taking advantage of recent advancements in biosensor technologies, we made spatially discrete near-real-time extracellular recordings of the major excitatory transmitter, glutamate, in the BLA of rats performing a self-paced lever-pressing sequence task for sucrose reward. This allowed us to detect rapid transient fluctuations in extracellular BLA glutamate time-locked to action performance. These glutamate transients tended to precede lever-pressing actions and were markedly increased in frequency when rats were engaged in such reward-seeking actions. Based on muscimol and tetrodotoxin microinfusions, these glutamate transients appeared to originate from the terminals of neurons with cell bodies in the orbital frontal cortex. Importantly, glutamate transient amplitude and frequency fluctuated with the value of the earned reward and positively predicted lever-pressing rate. Such novel rapid glutamate recordings during instrumental performance identify a role for glutamatergic signaling within the BLA in instrumental reward-seeking actions.
Figures






Similar articles
-
Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions.Sci Rep. 2015 Jul 27;5:12511. doi: 10.1038/srep12511. Sci Rep. 2015. PMID: 26212790 Free PMC article.
-
Contributions of basolateral amygdala and nucleus accumbens subregions to mediating motivational conflict during punished reward-seeking.Neurobiol Learn Mem. 2017 Apr;140:92-105. doi: 10.1016/j.nlm.2017.02.017. Epub 2017 Feb 24. Neurobiol Learn Mem. 2017. PMID: 28242266
-
Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats.Behav Neurosci. 2012 Aug;126(4):563-74. doi: 10.1037/a0029080. Behav Neurosci. 2012. PMID: 22845705 Free PMC article.
-
Valence-dependent contribution by the basolateral amygdala to active but not inhibitory avoidance and reward-seeking.Behav Brain Res. 2025 Apr 27;484:115503. doi: 10.1016/j.bbr.2025.115503. Epub 2025 Feb 20. Behav Brain Res. 2025. PMID: 39986615
-
The basolateral amygdala differentially regulates conditioned neural responses within the nucleus accumbens core and shell.Neuroscience. 2010 Sep 1;169(3):1186-98. doi: 10.1016/j.neuroscience.2010.05.073. Epub 2010 Jun 4. Neuroscience. 2010. PMID: 20570714 Free PMC article.
Cited by
-
A Micro-Platinum Wire Biosensor for Fast and Selective Detection of Alanine Aminotransferase.Sensors (Basel). 2016 May 26;16(6):767. doi: 10.3390/s16060767. Sensors (Basel). 2016. PMID: 27240366 Free PMC article.
-
Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery.Biosens Bioelectron. 2019 Apr 15;131:37-45. doi: 10.1016/j.bios.2019.01.060. Epub 2019 Feb 7. Biosens Bioelectron. 2019. PMID: 30818131 Free PMC article.
-
Intergenerational transmission of self-regulation: A multidisciplinary review and integrative conceptual framework.Psychol Bull. 2015 May;141(3):602-654. doi: 10.1037/a0038662. Psychol Bull. 2015. PMID: 25938878 Free PMC article. Review.
-
Dopamine's Effects on Corticostriatal Synapses during Reward-Based Behaviors.Neuron. 2018 Feb 7;97(3):494-510. doi: 10.1016/j.neuron.2018.01.006. Neuron. 2018. PMID: 29420932 Free PMC article. Review.
-
Effects of age and acute ethanol on glutamatergic neurotransmission in the medial prefrontal cortex of freely moving rats using enzyme-based microelectrode amperometry.PLoS One. 2015 Apr 30;10(4):e0125567. doi: 10.1371/journal.pone.0125567. eCollection 2015. PLoS One. 2015. PMID: 25927237 Free PMC article.
References
-
- Balleine B, Paredes-Olay C, Dickinson A. Effects of outcome devaluation on the performance of a heterogeneous instrumental chain. Int J Comp Psychol. 2005;18:257–272.
-
- Belay A, Collins A, Ruzgas T, Kissinger PT, Gorton L, Csöregi E. Redox hydrogel based bienzyme electrode for l-glutamate monitoring. J Pharm Biomed Anal. 1999;19:93–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
