Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons
- PMID: 22357858
- PMCID: PMC3305240
- DOI: 10.1523/JNEUROSCI.5135-11.2012
Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons
Abstract
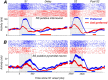
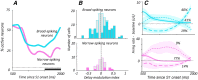
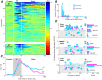
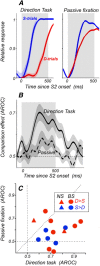
Comparing two stimuli that occur at different times demands the coordination of bottom-up and top-down processes. It has been hypothesized that the dorsolateral prefrontal (PFC) cortex, the likely source of top-down cortical influences, plays a key role in such tasks, contributing to both maintenance and sensory comparisons. We examined this hypothesis by recording from the PFC of monkeys comparing directions of two moving stimuli, S1 and S2, separated by a memory delay. We determined the contribution of the two principal cell types to these processes by classifying neurons into broad-spiking (BS) putative pyramidal cells and narrow-spiking (NS) putative local interneurons. During the delay, BS cells were more likely to exhibit anticipatory modulation and represent the remembered direction. While this representation was transient, appearing at different times in different neurons, it weakened when direction was not task relevant, suggesting its utility. During S2, both putative cell types showed comparison-related activity modulations. These modulations were of two types, each carried by different neurons, which either preferred trials with stimuli moving in the same direction or trials with stimuli of different directions. These comparison effects were strongly correlated with choice, suggesting their role in circuitry underlying decision making. These results provide the first demonstration of distinct contributions made by principal cell types to memory-guided perceptual decisions. During sensory stimulation both cell types represent behaviorally relevant stimulus features contributing to comparison and decision-related activity. However in the absence of sensory stimulation, putative pyramidal cells dominated, carrying information about the elapsed time and the preceding direction.
Figures




Similar articles
-
Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits.J Neurosci. 2016 Jan 13;36(2):489-505. doi: 10.1523/JNEUROSCI.3678-15.2016. J Neurosci. 2016. PMID: 26758840 Free PMC article.
-
Common rules guide comparisons of speed and direction of motion in the dorsolateral prefrontal cortex.J Neurosci. 2013 Jan 16;33(3):972-86. doi: 10.1523/JNEUROSCI.4075-12.2013. J Neurosci. 2013. PMID: 23325236 Free PMC article.
-
Flexibility of sensory representations in prefrontal cortex depends on cell type.Neuron. 2009 Dec 10;64(5):730-43. doi: 10.1016/j.neuron.2009.11.018. Neuron. 2009. PMID: 20005828 Free PMC article.
-
Prefrontal Neurons Represent Motion Signals from Across the Visual Field But for Memory-Guided Comparisons Depend on Neurons Providing These Signals.J Neurosci. 2016 Sep 7;36(36):9351-64. doi: 10.1523/JNEUROSCI.0843-16.2016. J Neurosci. 2016. PMID: 27605611 Free PMC article.
-
Linking Neuronal Direction Selectivity to Perceptual Decisions About Visual Motion.Annu Rev Vis Sci. 2020 Sep 15;6:335-362. doi: 10.1146/annurev-vision-121219-081816. Annu Rev Vis Sci. 2020. PMID: 32936737 Review.
Cited by
-
Persistent Spiking Activity Underlies Working Memory.J Neurosci. 2018 Aug 8;38(32):7020-7028. doi: 10.1523/JNEUROSCI.2486-17.2018. J Neurosci. 2018. PMID: 30089641 Free PMC article.
-
Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits.J Neurosci. 2016 Jan 13;36(2):489-505. doi: 10.1523/JNEUROSCI.3678-15.2016. J Neurosci. 2016. PMID: 26758840 Free PMC article.
-
Differences in reward processing between putative cell types in primate prefrontal cortex.PLoS One. 2017 Dec 19;12(12):e0189771. doi: 10.1371/journal.pone.0189771. eCollection 2017. PLoS One. 2017. PMID: 29261734 Free PMC article.
-
Contribution of Ionotropic Glutamatergic Receptors to Excitability and Attentional Signals in Macaque Frontal Eye Field.Cereb Cortex. 2021 Jun 10;31(7):3266-3284. doi: 10.1093/cercor/bhab007. Cereb Cortex. 2021. PMID: 33626129 Free PMC article.
-
Timing over tuning: overcoming the shortcomings of a line attractor during a working memory task.PLoS Comput Biol. 2014 Jan 30;10(1):e1003437. doi: 10.1371/journal.pcbi.1003437. eCollection 2014 Jan. PLoS Comput Biol. 2014. PMID: 24499929 Free PMC article.
References
-
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. - PubMed
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
