Isoform-specific toxicity of Mecp2 in postmitotic neurons: suppression of neurotoxicity by FoxG1
- PMID: 22357867
- PMCID: PMC3403752
- DOI: 10.1523/JNEUROSCI.5841-11.2012
Isoform-specific toxicity of Mecp2 in postmitotic neurons: suppression of neurotoxicity by FoxG1
Abstract
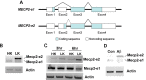
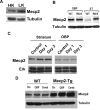
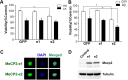
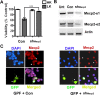
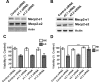
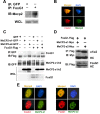
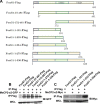
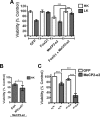
The methyl-CpG binding protein 2 (MeCP2) is a widely expressed protein, the mutations of which cause Rett syndrome. The level of MeCP2 is highest in the brain where it is expressed selectively in mature neurons. Its functions in postmitotic neurons are not known. The MeCP2 gene is alternatively spliced to generate two proteins with different N termini, designated as MeCP2-e1 and MeCP2-e2. The physiological significance of these two isoforms has not been elucidated, and it is generally assumed they are functionally equivalent. We report that in cultured cerebellar granule neurons induced to die by low potassium treatment and in Aβ-treated cortical neurons, Mecp2-e2 expression is upregulated whereas expression of the Mecp2-e1 isoform is downregulated. Knockdown of Mecp2-e2 protects neurons from death, whereas knockdown of the e1 isoform has no effect. Forced expression of MeCP2-e2, but not MeCP2-e1, promotes apoptosis in otherwise healthy neurons. We find that MeCP2-e2 interacts with the forkhead protein FoxG1, mutations of which also cause Rett syndrome. FoxG1 has been shown to promote neuronal survival and its downregulation leads to neuronal death. We find that elevated FoxG1 expression inhibits MeCP2-e2 neurotoxicity. MeCP2-e2 neurotoxicity is also inhibited by IGF-1, which prevents the neuronal death-associated downregulation of FoxG1 expression, and by Akt, the activation of which is necessary for FoxG1-mediated neuroprotection. Finally, MeCP2-e2 neurotoxicity is enhanced if FoxG1 expression is suppressed or in neurons cultured from FoxG1-haplodeficient mice. Our results indicate that Mecp2-e2 promotes neuronal death and that this activity is normally inhibited by FoxG1. Reduced FoxG1 expression frees MecP2-e2 to promote neuronal death.
Figures


Comment in
-
Insights into the cellular and molecular contributions of MeCP2 overexpression to disease pathophysiology.J Neurosci. 2012 Jul 11;32(28):9451-3. doi: 10.1523/JNEUROSCI.2043-12.2012. J Neurosci. 2012. PMID: 22787029 Free PMC article. No abstract available.
References
-
- Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, Boddaert N, Plouin P, Rio M, Fichou Y, Chelly J, Bienvenu T. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics. 2010;11:241–249. - PubMed
-
- Björkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET. All JNKs can kill, but nuclear localization is critical for neuronal death. J Biol Chem. 2008;283:19704–19713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
