Ubiquitin-dependent regulation of COPII coat size and function
- PMID: 22358839
- PMCID: PMC3292188
- DOI: 10.1038/nature10822
Ubiquitin-dependent regulation of COPII coat size and function
Abstract
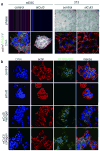
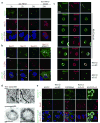
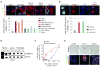
Packaging of proteins from the endoplasmic reticulum into COPII vesicles is essential for secretion. In cells, most COPII vesicles are approximately 60-80 nm in diameter, yet some must increase their size to accommodate 300-400 nm procollagen fibres or chylomicrons. Impaired COPII function results in collagen deposition defects, cranio-lenticulo-sutural dysplasia, or chylomicron retention disease, but mechanisms to enlarge COPII coats have remained elusive. Here, we identified the ubiquitin ligase CUL3-KLHL12 as a regulator of COPII coat formation. CUL3-KLHL12 catalyses the monoubiquitylation of the COPII-component SEC31 and drives the assembly of large COPII coats. As a result, ubiquitylation by CUL3-KLHL12 is essential for collagen export, yet less important for the transport of small cargo. We conclude that monoubiquitylation controls the size and function of a vesicle coat.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Cell biology: Collagen secretion explained.Nature. 2012 Feb 22;482(7386):474-5. doi: 10.1038/482474a. Nature. 2012. PMID: 22358830 Free PMC article.
-
COPII vesicles get supersized by ubiquitin.Cell. 2012 Mar 30;149(1):20-1. doi: 10.1016/j.cell.2012.03.006. Cell. 2012. PMID: 22464320
References
-
- Stephens LE, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

