Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages
- PMID: 22359457
- PMCID: PMC3284223
- DOI: 10.2147/IJN.S28531
Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages
Abstract
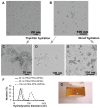
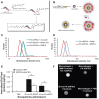
The assessment of macrophage response to nanoparticles is a central component in the evaluation of new nanoparticle designs for future in vivo application. This work investigates which feature, nanoparticle size or charge, is more predictive of non-specific uptake of nanoparticles by macrophages. This was investigated by synthesizing a library of polymer-coated iron oxide micelles, spanning a range of 30-100 nm in diameter and -23 mV to +9 mV, and measuring internalization into macrophages in vitro. Nanoparticle size and charge both contributed towards non-specific uptake, but within the ranges investigated, size appears to be a more dominant predictor of uptake. Based on these results, a protease-responsive nanoparticle was synthesized, displaying a matrix metalloproteinase-9 (MMP-9)-cleavable polymeric corona. These nanoparticles are able to respond to MMP-9 activity through the shedding of 10-20 nm of hydrodynamic diameter. This MMP-9-triggered decrease in nanoparticle size also led to up to a six-fold decrease in nanoparticle internalization by macrophages and is observable by T(2)-weighted magnetic resonance imaging. These findings guide the design of imaging or therapeutic nanoparticles for in vivo targeting of macrophage activity in pathologic states.
Keywords: iron oxides; macrophage targeting; opsonization; poly(ethylene glycol) (PEG); poly(propylene sulfide) (PPS).
Figures


Similar articles
-
Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol).Langmuir. 2006 Sep 12;22(19):8178-85. doi: 10.1021/la060951b. Langmuir. 2006. PMID: 16952259
-
Fluorescent magnetic nanoparticles with specific targeting functions for combinded targeting, optical imaging and magnetic resonance imaging.J Biomater Sci Polym Ed. 2012;23(15):1903-22. doi: 10.1163/092050611X598329. Epub 2012 May 8. J Biomater Sci Polym Ed. 2012. PMID: 22024467
-
Synthesis and functionalization of protease-activated nanoparticles with tissue plasminogen activator peptides as targeting moiety and diagnostic tool for pancreatic cancer.J Nanobiotechnology. 2016 Dec 19;14(1):81. doi: 10.1186/s12951-016-0236-3. J Nanobiotechnology. 2016. PMID: 27993133 Free PMC article.
-
Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture.Int J Mol Sci. 2012;13(5):5554-5570. doi: 10.3390/ijms13055554. Epub 2012 May 9. Int J Mol Sci. 2012. PMID: 22754315 Free PMC article.
-
Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): effect of poly(ethylene glycol) grafting density.Int J Pharm. 2011 Jun 15;411(1-2):178-87. doi: 10.1016/j.ijpharm.2011.02.039. Epub 2011 Mar 31. Int J Pharm. 2011. PMID: 21458551
Cited by
-
Antioxidant Response Activating nanoParticles (ARAPas) localize to atherosclerotic plaque and locally activate the Nrf2 pathway.Biomater Sci. 2022 Mar 2;10(5):1231-1247. doi: 10.1039/d1bm01421h. Biomater Sci. 2022. PMID: 35076645 Free PMC article.
-
Labelling primary immune cells using bright blue fluorescent nanoparticles.Biomater Sci. 2020 Mar 31;8(7):1897-1909. doi: 10.1039/c9bm01572h. Biomater Sci. 2020. PMID: 32026891 Free PMC article.
-
A review of nanotechnological approaches for the prophylaxis of HIV/AIDS.Biomaterials. 2013 Aug;34(26):6202-28. doi: 10.1016/j.biomaterials.2013.05.012. Epub 2013 May 28. Biomaterials. 2013. PMID: 23726227 Free PMC article. Review.
-
Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: noninvasive tracking using MRI.PLoS One. 2014 Mar 5;9(3):e90829. doi: 10.1371/journal.pone.0090829. eCollection 2014. PLoS One. 2014. PMID: 24598763 Free PMC article.
-
Actively Targeted Deep Tissue Imaging and Photothermal-Chemo Therapy of Breast Cancer by Antibody-Functionalized Drug-Loaded X-Ray-Responsive Bismuth Sulfide@Mesoporous Silica Core-Shell Nanoparticles.Adv Funct Mater. 2018 Jan 31;28(5):1704623. doi: 10.1002/adfm.201704623. Epub 2017 Dec 11. Adv Funct Mater. 2018. PMID: 29706855 Free PMC article.
References
-
- Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. - PubMed
-
- Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6(4):718–722. - PubMed
-
- Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op Mouse. J Bone Miner Res. 1990;5(7):781–789. - PubMed
-
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. - PubMed
-
- Linehan SA, Martinez-Pomares L, Gordon S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv Exp Med Biol. 2000;479:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

