Cigarette Smoke Extract-induced Reduction in Migration and Contraction in Normal Human Bronchial Smooth Muscle Cells
- PMID: 22359478
- PMCID: PMC3282228
- DOI: 10.4196/kjpp.2011.15.6.397
Cigarette Smoke Extract-induced Reduction in Migration and Contraction in Normal Human Bronchial Smooth Muscle Cells
Abstract
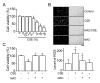
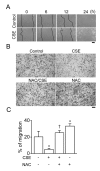
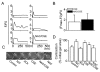
The proliferation, migration, cytokine release, and contraction of airway smooth muscle cells are key events in the airway remodeling process that occur in lung disease such as asthma, chronic obstruction pulmonary disease, and cancer. These events can be modulated by a number of factors, including cigarette smoke extract (CSE). CSE-induced alterations in the viability, migration, and contractile abilities of normal human airway cells remain unclear. This study investigated the effect of CSE on cell viability, migration, tumor necrosis factor (TNF)-α secretion, and contraction in normal human bronchial smooth muscle cells (HBSMCs). Treatment of HBSMCs with 10% CSE induced cell death, and the death was accompanied by the generation of reactive oxygen species (ROS). CSE-induced cell death was reduced by N-acetyl-l-cysteine (NAC), an ROS scavenger. In addition, CSE reduced the migration ability of HBSMCs by 75%. The combination of NAC with CSE blocked the CSE-induced reduction of cell migration. However, CSE had no effect on TNF-α secretion and NF-κB activation. CSE induced an increase in intracellular Ca(2+) concentration in 64% of HBSMCs. CSE reduced the contractile ability of HBSMCs, and the ability was enhanced by NAC treatment. These results demonstrate that CSE treatment induces cell death and reduces migration and contraction by increasing ROS generation in normal HBSMCs. These results suggest that CSE may induce airway change through cell death and reduction in migration and contraction of normal HBSMCs.
Keywords: Bronchiole; Cell migration; Cigarette smoke extract; Reactive oxygen species; Smooth muscle.
Figures
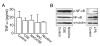
Similar articles
-
Aged red garlic extract reduces cigarette smoke extract-induced cell death in human bronchial smooth muscle cells by increasing intracellular glutathione levels.Phytother Res. 2012 Jan;26(1):18-25. doi: 10.1002/ptr.3502. Epub 2011 Apr 28. Phytother Res. 2012. PMID: 21538625
-
Inflammatory Effects of Menthol vs. Non-menthol Cigarette Smoke Extract on Human Lung Epithelial Cells: A Double-Hit on TRPM8 by Reactive Oxygen Species and Menthol.Front Physiol. 2017 Apr 27;8:263. doi: 10.3389/fphys.2017.00263. eCollection 2017. Front Physiol. 2017. PMID: 28496415 Free PMC article.
-
Periostin mediates cigarette smoke extract-induced proliferation and migration in pulmonary arterial smooth muscle cells.Biomed Pharmacother. 2016 Oct;83:514-520. doi: 10.1016/j.biopha.2016.07.007. Epub 2016 Jul 18. Biomed Pharmacother. 2016. PMID: 27434868
-
Cigarette smoke extract inhibits cell migration and contraction via the reactive oxygen species/adenosine monophosphate-activated protein kinase pathway in nasal fibroblasts.Int Forum Allergy Rhinol. 2020 Mar;10(3):356-363. doi: 10.1002/alr.22479. Epub 2019 Nov 6. Int Forum Allergy Rhinol. 2020. PMID: 31693801
-
Cigarette smoke and α,β-unsaturated aldehydes elicit VEGF release through the p38 MAPK pathway in human airway smooth muscle cells and lung fibroblasts.Br J Pharmacol. 2011 Jun;163(3):649-61. doi: 10.1111/j.1476-5381.2011.01253.x. Br J Pharmacol. 2011. PMID: 21306579 Free PMC article.
Cited by
-
Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells.BMC Pulm Med. 2014 Mar 1;14:32. doi: 10.1186/1471-2466-14-32. BMC Pulm Med. 2014. PMID: 24581246 Free PMC article.
-
In vitro biological assessment of the stability of cigarette smoke aqueous aerosol extracts.BMC Res Notes. 2020 Oct 21;13(1):492. doi: 10.1186/s13104-020-05337-2. BMC Res Notes. 2020. PMID: 33087173 Free PMC article.
-
Acute cigarette smoke or extract exposure rapidly activates TRPA1-mediated calcium influx in primary human airway smooth muscle cells.Sci Rep. 2021 May 5;11(1):9643. doi: 10.1038/s41598-021-89051-4. Sci Rep. 2021. PMID: 33953304 Free PMC article.
-
Modelling the asthma phenotype: impact of cigarette smoke exposure.Respir Res. 2018 May 10;19(1):89. doi: 10.1186/s12931-018-0799-7. Respir Res. 2018. PMID: 29747661 Free PMC article.
-
Potential Utilisation of Theobroma cacao Pod Husk Extract: Protective Capability Evaluation Against Pollution Models and Formulation into Niosomes.Trop Life Sci Res. 2024 Jul;35(2):107-140. doi: 10.21315/tlsr2024.35.2.6. Epub 2024 Jul 31. Trop Life Sci Res. 2024. PMID: 39234471 Free PMC article.
References
-
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. - PubMed
-
- Tang ML, Wilson JW, Stewart AG, Royce SG. Airway remodelling in asthma: current understanding and implications for future therapies. Pharmacol Ther. 2006;112:474–488. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

