Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence
- PMID: 22359501
- PMCID: PMC3280987
- DOI: 10.1371/journal.ppat.1002518
Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence
Abstract
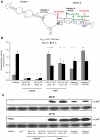
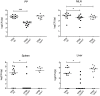
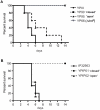
Expression of all Yersinia pathogenicity factors encoded on the virulence plasmid, including the yop effector and the ysc type III secretion genes, is controlled by the transcriptional activator LcrF in response to temperature. Here, we show that a protein- and RNA-dependent hierarchy of thermosensors induce LcrF synthesis at body temperature. Thermally regulated transcription of lcrF is modest and mediated by the thermo-sensitive modulator YmoA, which represses transcription from a single promoter located far upstream of the yscW-lcrF operon at moderate temperatures. The transcriptional response is complemented by a second layer of temperature-control induced by a unique cis-acting RNA element located within the intergenic region of the yscW-lcrF transcript. Structure probing demonstrated that this region forms a secondary structure composed of two stemloops at 25°C. The second hairpin sequesters the lcrF ribosomal binding site by a stretch of four uracils. Opening of this structure was favored at 37°C and permitted ribosome binding at host body temperature. Our study further provides experimental evidence for the biological relevance of an RNA thermometer in an animal model. Following oral infections in mice, we found that two different Y. pseudotuberculosis patient isolates expressing a stabilized thermometer variant were strongly reduced in their ability to disseminate into the Peyer's patches, liver and spleen and have fully lost their lethality. Intriguingly, Yersinia strains with a destabilized version of the thermosensor were attenuated or exhibited a similar, but not a higher mortality. This illustrates that the RNA thermometer is the decisive control element providing just the appropriate amounts of LcrF protein for optimal infection efficiency.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



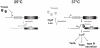
References
-
- Bottone EJ. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1999;1:323–333. - PubMed
-
- Dube P. Interaction of Yersinia with the gut: mechanisms of pathogenesis and immune evasion. Curr Top Microbiol Immunol. 2009;337:61–91. - PubMed
-
- Straley SC, Perry RD. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. - PubMed
-
- Revell PA, Miller VL. Yersinia virulence: more than a plasmid. FEMS Microbiol Lett. 2001;205:159–164. - PubMed
-
- Heroven AK, Bohme K, Tran-Winkler H, Dersch P. Regulatory elements implicated in the environmental control of invasin expression in enteropathogenic Yersinia. Adv Exp Med Biol. 2007;603:156–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

