Decoding the distribution of glycan receptors for human-adapted influenza A viruses in ferret respiratory tract
- PMID: 22359533
- PMCID: PMC3281014
- DOI: 10.1371/journal.pone.0027517
Decoding the distribution of glycan receptors for human-adapted influenza A viruses in ferret respiratory tract
Abstract
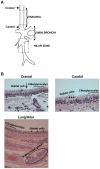
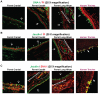
Ferrets are widely used as animal models for studying influenza A viral pathogenesis and transmissibility. Human-adapted influenza A viruses primarily target the upper respiratory tract in humans (infection of the lower respiratory tract is observed less frequently), while in ferrets, upon intranasal inoculation both upper and lower respiratory tract are targeted. Viral tropism is governed by distribution of complex sialylated glycan receptors in various cells/tissues of the host that are specifically recognized by influenza A virus hemagglutinin (HA), a glycoprotein on viral surface. It is generally known that upper respiratory tract of humans and ferrets predominantly express α2→6 sialylated glycan receptors. However much less is known about the fine structure of these glycan receptors and their distribution in different regions of the ferret respiratory tract. In this study, we characterize distribution of glycan receptors going beyond terminal sialic acid linkage in the cranial and caudal regions of the ferret trachea (upper respiratory tract) and lung hilar region (lower respiratory tract) by multiplexing use of various plant lectins and human-adapted HAs to stain these tissue sections. Our findings show that the sialylated glycan receptors recognized by human-adapted HAs are predominantly distributed in submucosal gland of lung hilar region as a part of O-linked glycans. Our study has implications in understanding influenza A viral pathogenesis in ferrets and also in employing ferrets as animal models for developing therapeutic strategies against influenza.
Conflict of interest statement
Figures


References
-
- Maher JA, DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim (NY) 2004;33:50–53. - PubMed
-
- Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

