Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells
- PMID: 22359538
- PMCID: PMC3281009
- DOI: 10.1371/journal.pone.0029727
Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells
Abstract
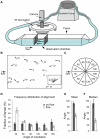
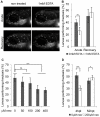
The lateral line sensory system, found in fish and amphibians, is used in prey detection, predator avoidance and schooling behavior. This system includes cell clusters, called superficial neuromasts, located on the surface of head and trunk of developing larvae. Mechanosensory hair cells in the center of each neuromast respond to disturbances in the water and convey information to the brain via the lateral line ganglia. The convenient location of mechanosensory hair cells on the body surface has made the lateral line a valuable system in which to study hair cell damage and regeneration. One way to measure hair cell survival and recovery is to assay behaviors that depend on their function. We built a system in which orientation against constant water flow, positive rheotaxis, can be quantitatively assessed. We found that zebrafish larvae perform positive rheotaxis and that, similar to adult fish, larvae use both visual and lateral line input to perform this behavior. Disruption or damage of hair cells in the absence of vision leads to a marked decrease in rheotaxis that recovers upon hair cell repair or regeneration.
Conflict of interest statement
Figures

References
-
- Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. - PubMed
-
- Metcalfe WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. - PubMed
-
- Partridge BL, Pitcher TL. The sensory basis of fish schools; relative roles of lateral line and vision. J Comp Physiol. 1980;135:315–325.
-
- Hoekstra D, Janssen J. Non-visual feeding behaviour of the mottled sculpin, Cottus bairdi in Lake Michigan. Env Biol Fish. 1985;12:111–117.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

