Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R
- PMID: 22359543
- PMCID: PMC3281035
- DOI: 10.1371/journal.pone.0030512
Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R
Erratum in
-
Correction: Improved Control of Tuberculosis and Activation of Macrophages in Mice Lacking Protein Kinase R.PLoS One. 2018 Oct 5;13(10):e0205424. doi: 10.1371/journal.pone.0205424. eCollection 2018. PLoS One. 2018. PMID: 30289942 Free PMC article.
Abstract
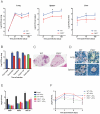
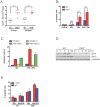
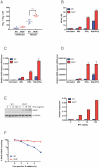
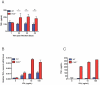
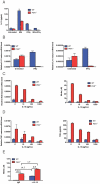
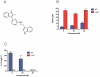
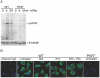
Host factors that microbial pathogens exploit for their propagation are potential targets for therapeuic countermeasures. No host enzyme has been identified whose genetic absence benefits the intact mammalian host in vivo during infection with Mycobacterium tuberculosis (Mtb), the leading cause of death from bacterial infection. Here, we report that the dsRNA-dependent protein kinase (PKR) is such an enzyme. PKR-deficient mice contained fewer viable Mtb and showed less pulmonary pathology than wild type mice. We identified two potential mechanisms for the protective effect of PKR deficiency: increased apoptosis of macrophages in response to Mtb and enhanced activation of macrophages in response to IFN-gamma. The restraining effect of PKR on macrophage activation was explained by its mediation of a previously unrecognized ability of IFN-gamma to induce low levels of the macrophage deactivating factor interleukin 10 (IL10). These observations suggest that PKR inhibitors may prove useful as an adjunctive treatment for tuberculosis.
Conflict of interest statement
Figures
References
-
- Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens. Sci Signal. 2008;1:re8. - PubMed
-
- Cohen SN. Microbial drug resistance: An old problem in need of new solutions. In: Relman DA, Hamburg MA, Choffnes EE, Mack A, editors. Microbial Evolution and Co-Adaptation:A Tribute to the Life and Scientific Legacies of Joshua Lederberg. National Academies Press; 2009. pp. 173–180. 2009; Washington, DC. - PubMed
-
- Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. - PubMed
-
- Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

