Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts
- PMID: 22359546
- PMCID: PMC3281033
- DOI: 10.1371/journal.pone.0030606
Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts
Abstract
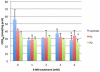
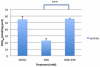
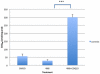
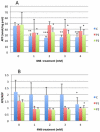
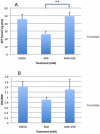
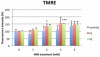
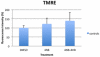
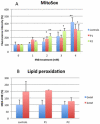
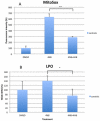
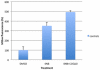
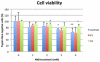
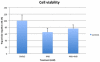
Coenzyme Q(10) (CoQ(10)) is a potent lipophilic antioxidant in cell membranes and a carrier of electrons in the mitochondrial respiratory chain. We previously characterized the effects of varying severities of CoQ(10) deficiency on ROS production and mitochondrial bioenergetics in cells harboring genetic defects of CoQ(10) biosynthesis. We observed a unimodal distribution of ROS production with CoQ(10) deficiency: cells with <20% of CoQ(10) and 50-70% of CoQ(10) did not generate excess ROS while cells with 30-45% of CoQ(10) showed increased ROS production and lipid peroxidation. Because our previous studies were limited to a small number of mutant cell lines with heterogeneous molecular defects, here, we treated 5 control and 2 mildly CoQ(10) deficient fibroblasts with varying doses of 4-nitrobenzoate (4-NB), an analog of 4-hydroxybenzoate (4-HB) and inhibitor of 4-para-hydroxybenzoate:polyprenyl transferase (COQ2) to induce a range of CoQ(10) deficiencies. Our results support the concept that the degree of CoQ(10) deficiency in cells dictates the extent of ATP synthesis defects and ROS production and that 40-50% residual CoQ(10) produces maximal oxidative stress and cell death.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

