Morphine suppresses IFN signaling pathway and enhances AIDS virus infection
- PMID: 22359571
- PMCID: PMC3281044
- DOI: 10.1371/journal.pone.0031167
Morphine suppresses IFN signaling pathway and enhances AIDS virus infection
Abstract
Background: Opioids exert a profound influence on immunomodulation and enhance HIV infection and replication. However, the mechanism(s) of their action remains to be determined. We thus investigated the impact of morphine on the intracellular innate antiviral immunity.
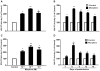
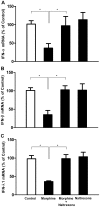
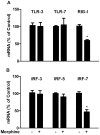
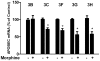
Methodology/principal findings: Seven-day-cultured macrophages were infected with equal amounts of cell-free HIV Bal or SIV Delta(B670) for 2 h at 37°C after 24 h of treatment with or without morphine. Effect of morphine on HIV/SIV infection and replication was evaluated by HIV/SIV RT activity assay and indirect immunofluorescence for HIV p24 or SIV p28 antigen. The mRNA expression of cellular factors suppressed or induced by morphine treatment was analyzed by the real-time RT-PCR. We demonstrated that morphine treatment of human blood monocyte-derived macrophages significantly inhibited the expression of interferons (IFN-α, IFN-β and IFN-λ) and IFN-inducible genes (APOBEC3C/3F/3G and 3H). The further experiments showed that morphine suppressed the expression of several key elements (RIG-I and IRF-7) in IFN signaling pathway. In addition, morphine treatment induced the expression of suppressor of cytokine signaling protein-1, 2, 3 (SOCS-1, 2, 3) and protein inhibitors of activated STAT-1, 3, X, Y (PIAS-1, 3, X, Y), the key negative regulators of IFN signaling pathway.
Conclusions: These findings indicate that morphine impairs intracellular innate antiviral mechanism(s) in macrophages, contributing to cell susceptibility to AIDS virus infection.
Conflict of interest statement
Figures



Similar articles
-
Modulation of intracellular restriction factors contributes to methamphetamine-mediated enhancement of acquired immune deficiency syndrome virus infection of macrophages.Curr HIV Res. 2012 Jul;10(5):407-14. doi: 10.2174/157016212802138797. Curr HIV Res. 2012. PMID: 22591364 Free PMC article. Review.
-
Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages.J Immunol. 2010 Aug 15;185(4):2393-404. doi: 10.4049/jimmunol.0903563. Epub 2010 Jul 14. J Immunol. 2010. PMID: 20631305 Free PMC article.
-
IFN-λ3 inhibits HIV infection of macrophages through the JAK-STAT pathway.PLoS One. 2012;7(4):e35902. doi: 10.1371/journal.pone.0035902. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558263 Free PMC article.
-
Comparison of antiviral activity of lambda-interferons against HIV replication in macrophages.J Interferon Cytokine Res. 2015 Mar;35(3):213-21. doi: 10.1089/jir.2014.0064. Epub 2014 Sep 30. J Interferon Cytokine Res. 2015. PMID: 25268605 Free PMC article.
-
Manipulating the Interferon Signaling Pathway: Implications for HIV Infection.Virol Sin. 2019 Apr;34(2):192-196. doi: 10.1007/s12250-019-00085-5. Epub 2019 Feb 14. Virol Sin. 2019. PMID: 30762199 Free PMC article. Review.
Cited by
-
Angiotensin converting enzyme-2 as therapeutic target in COVID-19.Diabetes Metab Syndr. 2020 Jul-Aug;14(4):637-639. doi: 10.1016/j.dsx.2020.05.022. Epub 2020 May 12. Diabetes Metab Syndr. 2020. PMID: 32428864 Free PMC article.
-
Age differences in cytokine expression under conditions of health using experimental pain models.Exp Gerontol. 2015 Dec;72:150-6. doi: 10.1016/j.exger.2015.09.017. Epub 2015 Oct 9. Exp Gerontol. 2015. PMID: 26456458 Free PMC article.
-
The Effects of Opium Addiction on the Immune System Function in Patients with Fungal Infection.Addict Health. 2016 Fall;8(4):218-226. Addict Health. 2016. PMID: 28819552 Free PMC article.
-
Effects of Morphine on Gp120-induced Neuroinflammation Under Immunocompetent Vs. Immunodeficient Conditions.J Neuroimmune Pharmacol. 2023 Jun;18(1-2):24-40. doi: 10.1007/s11481-021-10040-5. Epub 2022 Jan 21. J Neuroimmune Pharmacol. 2023. PMID: 35059975
-
Morphine-potentiated cognitive deficits correlate to suppressed hippocampal iNOS RNA expression and an absent type 1 interferon response in LP-BM5 murine AIDS.J Neuroimmunol. 2018 Jun 15;319:117-129. doi: 10.1016/j.jneuroim.2018.02.017. Epub 2018 Mar 6. J Neuroimmunol. 2018. PMID: 29526406 Free PMC article.
References
-
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83:4–18. - PubMed
-
- Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–1479. - PubMed
-
- Ronald PJ, Robertson JR, Elton RA. Continued drug use and other cofactors for progression to AIDS among injecting drug users. AIDS. 1994;8:339–343. - PubMed
-
- Battjes RJ, Leukefeld CG, Pickens RW, Haverkos HW. The acquired immunodeficiency syndrome and intravenous drug abuse. Bull Narc. 1988;40:21–34. - PubMed
-
- Specter S. Drugs of abuse and infectious diseases. J Fla Med Assoc. 1994;81:485–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

