The functions of myosin II and myosin V homologs in tip growth and septation in Aspergillus nidulans
- PMID: 22359575
- PMCID: PMC3281053
- DOI: 10.1371/journal.pone.0031218
The functions of myosin II and myosin V homologs in tip growth and septation in Aspergillus nidulans
Abstract
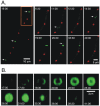
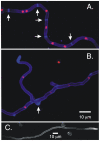
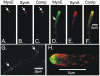
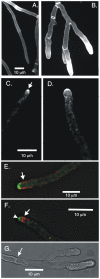
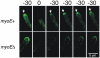
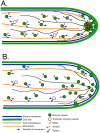
Because of the industrial and medical importance of members of the fungal genus Aspergillus, there is considerable interest in the functions of cytoskeletal components in growth and secretion in these organisms. We have analyzed the genome of Aspergillus nidulans and found that there are two previously unstudied myosin genes, a myosin II homolog, myoB (product = MyoB) and a myosin V homolog, myoE (product = MyoE). Deletions of either cause significant growth defects. MyoB localizes in strings that coalesce into contractile rings at forming septa. It is critical for septation and normal deposition of chitin but not for hyphal extension. MyoE localizes to the Spitzenkörper and to moving puncta in the cytoplasm. Time-lapse imaging of SynA, a v-SNARE, reveals that in myoE deletion strains vesicles no longer localize to the Spitzenkörper. Tip morphology is slightly abnormal and branching occurs more frequently than in controls. Tip extension is slower than in controls, but because hyphal diameter is greater, growth (increase in volume/time) is only slightly reduced. Concentration of vesicles into the Spitzenkörper before incorporation into the plasma membrane is, thus, not required for hyphal growth but facilitates faster tip extension and a more normal hyphal shape.
Conflict of interest statement
Figures



References
-
- Wolkow TD, Harris SD, Hamer JE. Cytokinesis in Aspergillus nidulans is controlled by cell size, nuclear positioning and mitosis. J Cell Sci. 1996;109:2179–2188. - PubMed
-
- Yamashita RA, May GS. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. J Biol Chem. 1998;273:14644–14648. - PubMed
-
- Yamashita RA, Osherov N, May GS. Localization of wild type and mutant class I myosin proteins in Aspergillus nidulans using GFP-fusion proteins. Cell Motil Cytoskeleton. 2000;45:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

