Impact of the herbal medicine Sophora flavescens on the oral pharmacokinetics of indinavir in rats: the involvement of CYP3A and P-glycoprotein
- PMID: 22359586
- PMCID: PMC3281083
- DOI: 10.1371/journal.pone.0031312
Impact of the herbal medicine Sophora flavescens on the oral pharmacokinetics of indinavir in rats: the involvement of CYP3A and P-glycoprotein
Abstract
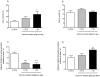
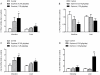
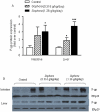
Sophora flavescens is a Chinese medicinal herb used for the treatment of gastrointestinal hemorrhage, skin diseases, pyretic stranguria and viral hepatitis. In this study the herb-drug interactions between S. flavescens and indinavir, a protease inhibitor for HIV treatment, were evaluated in rats. Concomitant oral administration of Sophora extract (0.158 g/kg or 0.63 g/kg, p.o.) and indinavir (40 mg/kg, p.o.) in rats twice a day for 7 days resulted in a dose-dependent decrease of plasma indinavir concentrations, with 55%-83% decrease in AUC(0-∞) and 38%-78% reduction in C(max). The CL (Clearance)/F (fraction of dose available in the systemic circulation) increased up to 7.4-fold in Sophora-treated rats. Oxymatrine treatment (45 mg/kg, p.o.) also decreased indinavir concentrations, while the ethyl acetate fraction of Sophora extract had no effect. Urinary indinavir (24-h) was reduced, while the fraction of indinavir in faeces was increased after Sophora treatment. Compared to the controls, multiple dosing of Sophora extract elevated both mRNA and protein levels of P-gp in the small intestine and liver. In addition, Sophora treatment increased intestinal and hepatic mRNA expression of CYP3A1, but had less effect on CYP3A2 expression. Although protein levels of CYP3A1 and CYP3A2 were not altered by Sophora treatment, hepatic CYP3A activity increased in the Sophora-treated rats. All available data demonstrated that Sophora flavescens reduced plasma indinavir concentration after multiple concomitant doses, possibly through hepatic CYP3A activity and induction of intestinal and hepatic P-gp. The animal study would be useful for predicting potential interactions between natural products and oral pharmaceutics and understanding the mechanisms prior to human studies. Results in the current study suggest that patients using indinavir might be cautioned in the use of S. flavescens extract or Sophora-derived products.
Conflict of interest statement
Figures



Similar articles
-
Sophora flavescens (Ku-Shen) as a booster for antiretroviral therapy through cytochrome P450 3A4 inhibition.Hong Kong Med J. 2015 Dec;21 Suppl 7:S18-21. Hong Kong Med J. 2015. PMID: 26908268 No abstract available.
-
Dextran sulfate sodium-induced colitis and ginseng intervention altered oral pharmacokinetics of cyclosporine A in rats.J Ethnopharmacol. 2021 Jan 30;265:113251. doi: 10.1016/j.jep.2020.113251. Epub 2020 Aug 15. J Ethnopharmacol. 2021. PMID: 32810615
-
Effects of St. John's wort extract on indinavir pharmacokinetics in rats: differentiation of intestinal and hepatic impacts.Life Sci. 2009 Aug 12;85(7-8):296-302. doi: 10.1016/j.lfs.2009.06.008. Epub 2009 Jun 24. Life Sci. 2009. PMID: 19559714
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience.Antivir Ther. 2001 Dec;6(4):201-29. Antivir Ther. 2001. PMID: 11878403 Review.
Cited by
-
Understanding the relevance of herb-drug interaction studies with special focus on interplays: a prerequisite for integrative medicine.Porto Biomed J. 2019 Mar 1;4(2):e15. doi: 10.1016/j.pbj.0000000000000015. eCollection 2019 Mar-Apr. Porto Biomed J. 2019. PMID: 31595257 Free PMC article. Review.
-
Contribution of baicalin on the plasma protein binding displacement and CYP3A activity inhibition to the pharmacokinetic changes of nifedipine in rats in vivo and in vitro.PLoS One. 2014 Jan 30;9(1):e87234. doi: 10.1371/journal.pone.0087234. eCollection 2014. PLoS One. 2014. PMID: 24498050 Free PMC article. Clinical Trial.
-
The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury.Front Pharmacol. 2016 Jan 14;6:326. doi: 10.3389/fphar.2015.00326. eCollection 2015. Front Pharmacol. 2016. PMID: 26834638 Free PMC article.
-
Inhibition of Cytochrome P450 Activities by Sophora flavescens Extract and Its Prenylated Flavonoids in Human Liver Microsomes.Evid Based Complement Alternat Med. 2019 Mar 13;2019:2673769. doi: 10.1155/2019/2673769. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31001351 Free PMC article.
-
Phytochemicals That Interfere With Drug Metabolism and Transport, Modifying Plasma Concentration in Humans and Animals.Dose Response. 2022 Sep 21;20(3):15593258221120485. doi: 10.1177/15593258221120485. eCollection 2022 Jul-Sep. Dose Response. 2022. PMID: 36158743 Free PMC article. Review.
References
-
- National Committee of Chinese Pharmacopoeia. Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press; 2010. pp. 61–62.
-
- Chen X, Yi C, Yang X, Wang X. Liquid chromatography of active principles in Sophora flavescens root. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:149–163. - PubMed
-
- Zhang L, Xu L, Xiao SS, Liao QF, Li Q, et al. Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2007;44:1019–1028. - PubMed
-
- Sun M, Han J, Duan J, Cui Y, Wang T, et al. Novel antitumor activities of Kushen flavonoids in vitro and in vivo. Phytother Res. 2007;21:269–277. - PubMed
-
- Sun H, Li L, Shang L, Zhao D, Dong D, et al. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res. 2008;22:985–989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

