Endothelial microparticles (EMP) for the assessment of endothelial function: an in vitro and in vivo study on possible interference of plasma lipids
- PMID: 22359595
- PMCID: PMC3281080
- DOI: 10.1371/journal.pone.0031496
Endothelial microparticles (EMP) for the assessment of endothelial function: an in vitro and in vivo study on possible interference of plasma lipids
Abstract
Background: Circulating endothelial microparticles (EMP) reflect the condition of the endothelium and are of increasing interest in cardiovascular and inflammatory diseases. Recently, increased numbers of EMP following oral fat intake, possibly due to acute endothelial injury, have been reported. On the other hand, the direct interference of lipids with the detection of EMP has been suggested. This study aimed to investigate the effect of lipid-rich solutions, commonly administered in clinical practice, on the detection, both in vitro and in vivo, of EMP.
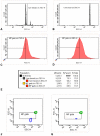
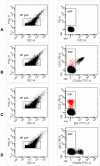
Methods: For the in vitro assessment, several lipid-rich solutions were added to whole blood of healthy subjects (n = 8) and patients with coronary heart disease (n = 5). EMP (CD31+/CD42b-) were detected in platelet poor plasma by flow cytometry. For the in vivo study, healthy volunteers were evaluated on 3 different study-days: baseline evaluation, following lipid infusion and after a NaCl infusion. EMP quantification, lipid measurements and peripheral arterial tonometry were performed on each day.
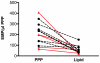
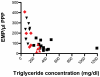
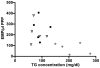
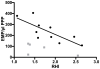
Results: Both in vitro addition and in vivo administration of lipids significantly decreased EMP (from 198.6 to 53.0 and from 272.6 to 90.6/µl PPP, respectively, p = 0.001 and p = 0.012). The EMP number correlated inversely with the concentration of triglycerides, both in vitro and in vivo (r = -0.707 and -0.589, p<0.001 and p = 0.021, respectively). The validity of EMP as a marker of endothelial function is supported by their inverse relationship with the reactive hyperemia index (r = -0.758, p = 0.011). This inverse relation was confounded by the intravenous administration of lipids.
Conclusion: The confounding effect of high circulating levels of lipids, commonly found in patients that receive intravenous lipid-based solutions, should be taken into account when flow cytometry is used to quantify EMP.
Conflict of interest statement
Figures

Similar articles
-
Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients.Br J Nutr. 2014 Apr 14;111(7):1245-52. doi: 10.1017/S0007114513003693. Epub 2013 Nov 29. Br J Nutr. 2014. PMID: 24286443 Clinical Trial.
-
Endothelial microparticles correlate with erectile dysfunction in diabetic men.Int J Impot Res. 2007 Mar-Apr;19(2):161-6. doi: 10.1038/sj.ijir.3901500. Epub 2006 Aug 10. Int J Impot Res. 2007. PMID: 16900206
-
Elevated plasma endothelial microparticles in multiple sclerosis.Neurology. 2001 May 22;56(10):1319-24. doi: 10.1212/wnl.56.10.1319. Neurology. 2001. PMID: 11376181
-
Endothelial microparticles in diseases.Cell Tissue Res. 2009 Jan;335(1):143-51. doi: 10.1007/s00441-008-0710-9. Epub 2008 Nov 7. Cell Tissue Res. 2009. PMID: 18989704 Review.
-
Endothelial microparticles as markers of endothelial dysfunction.Front Biosci. 2004 May 1;9:1118-35. doi: 10.2741/1270. Front Biosci. 2004. PMID: 14977533 Review.
Cited by
-
Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction.Biomedicines. 2024 Sep 18;12(9):2119. doi: 10.3390/biomedicines12092119. Biomedicines. 2024. PMID: 39335632 Free PMC article.
-
Extracellular vesicles in atherothrombosis: From biomarkers and precision medicine to therapeutic targets.Immunol Rev. 2022 Nov;312(1):6-19. doi: 10.1111/imr.13127. Epub 2022 Aug 22. Immunol Rev. 2022. PMID: 35996799 Free PMC article. Review.
-
Methods for extracellular vesicles isolation in a hospital setting.Front Immunol. 2015 Feb 13;6:50. doi: 10.3389/fimmu.2015.00050. eCollection 2015. Front Immunol. 2015. PMID: 25762995 Free PMC article.
-
Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis.Cells. 2022 Jun 5;11(11):1845. doi: 10.3390/cells11111845. Cells. 2022. PMID: 35681540 Free PMC article. Review.
-
Could Microparticles Be the Universal Quality Indicator for Platelet Viability and Function?J Blood Transfus. 2016;2016:6140239. doi: 10.1155/2016/6140239. Epub 2016 Dec 8. J Blood Transfus. 2016. PMID: 28053805 Free PMC article. Review.
References
-
- Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–1135. - PubMed
-
- Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:27–33. - PubMed
-
- Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biro E, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–1851. - PubMed
-
- Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio Journal of Medicine. 2004;53:210–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

