The orphan nuclear receptor LRH-1 and ERα activate GREB1 expression to induce breast cancer cell proliferation
- PMID: 22359603
- PMCID: PMC3281101
- DOI: 10.1371/journal.pone.0031593
The orphan nuclear receptor LRH-1 and ERα activate GREB1 expression to induce breast cancer cell proliferation
Abstract
Background: Liver Receptor Homolog 1 (LRH-1, NR5A2) is an orphan nuclear receptor that is over-expressed in cancers in tissues such as the breast, colon and pancreas. LRH-1 plays important roles in embryonic development, steroidogenesis and cholesterol homeostasis. In tumor cells, LRH-1 induces proliferation and cell cycle progression. High LRH-1 expression is demonstrated in breast cancers, positively correlating with ERα status and aromatase activity. LRH-1 dependent cellular mechanisms in breast cancer epithelial cells are poorly defined. Hence in the present study we investigated the actions of LRH-1 in estrogen receptor α (ERα) positive breast cancer cells.
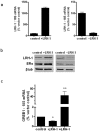
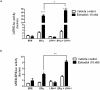
Results: The study aimed to investigate LRH-1 dependent mechanisms that promote breast cancer proliferation. We identified that LRH-1 regulated the expression of Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in MCF-7 and MDA-MB-231 cells. Over-expression of LRH-1 increased GREB1 mRNA levels while knockdown of LRH-1 reduced its expression. GREB1 is a well characterised ERα target gene, with three estrogen response elements (ERE) located on its promoter. Chromatin immunoprecipitation studies provided evidence of the co-localisation of LRH-1 and ERα at all three EREs. With electrophoretic mobility shift assays, we demonstrated direct binding of LRH-1 to EREs located on GREB1 and Trefoil Factor 1 (TFF1, pS2) promoters. LRH-1 and ERα co-operatively activated transcription of ERE luciferase reporter constructs suggesting an overlap in regulation of target genes in breast cancer cells. Over-expression of LRH-1 resulted in an increase in cell proliferation. This effect was more pronounced with estradiol treatment. In the presence of ICI 182,780, an ERα antagonist, LRH-1 still induced proliferation.
Conclusions: We conclude that in ER-positive breast cancer cells, LRH-1 promotes cell proliferation by enhancing ERα mediated transcription of target genes such as GREB-1. Collectively these findings indicate the importance of LRH-1 in the progression of hormone-dependent breast cancer and implicate LRH-1 as a potential avenue for drug development.
Conflict of interest statement
Figures




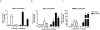
References
-
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. - PubMed
-
- Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. - PubMed
-
- Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

