Collective motion of cells mediates segregation and pattern formation in co-cultures
- PMID: 22359617
- PMCID: PMC3280994
- DOI: 10.1371/journal.pone.0031711
Collective motion of cells mediates segregation and pattern formation in co-cultures
Abstract
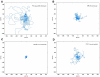
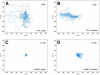
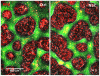
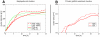
Pattern formation by segregation of cell types is an important process during embryonic development. We show that an experimentally yet unexplored mechanism based on collective motility of segregating cells enhances the effects of known pattern formation mechanisms such as differential adhesion, mechanochemical interactions or cell migration directed by morphogens. To study in vitro cell segregation we use time-lapse videomicroscopy and quantitative analysis of the main features of the motion of individual cells or groups. Our observations have been extensive, typically involving the investigation of the development of patterns containing up to 200,000 cells. By either comparing keratocyte types with different collective motility characteristics or increasing cells' directional persistence by the inhibition of Rac1 GTP-ase we demonstrate that enhanced collective cell motility results in faster cell segregation leading to the formation of more extensive patterns. The growth of the characteristic scale of patterns generally follows an algebraic scaling law with exponent values up to 0.74 in the presence of collective motion, compared to significantly smaller exponents in case of diffusive motion.
Conflict of interest statement
Figures




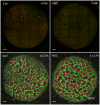
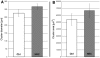

References
-
- Turing AM. The chemical basis of morphogenesis, Phil. Trans R Soc (Lond.) 1952;B237:37–72.
-
- Murray JD. On the mechanochemical theory of biological pattern formation with application to vasculogenesis. C R Biol. 2003;326:239–252. - PubMed
-
- Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. - PubMed
-
- Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet. 2007;Dev17:281–286. - PubMed
-
- Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–1620. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

