Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells
- PMID: 22359647
- PMCID: PMC3281086
- DOI: 10.1371/journal.pone.0031962
Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells
Abstract
Background: Cancer vaccines are designed to activate and enhance cancer-antigen-targeted T cells that are suppressed through multiple mechanisms of immune tolerance in cancer-bearing hosts. T regulatory cell (Treg) suppression of tumor-specific T cells is one barrier to effective immunization. A second mechanism is the deletion of high avidity tumor-specific T cells, which leaves a less effective low avidity tumor specific T cell repertoire available for activation by vaccines. Treg depleting agents including low dose cyclophosphamide (Cy) and antibodies that deplete CD25-expressing Tregs have been used with limited success to enhance the potency of tumor-specific vaccines. In addition, few studies have evaluated mechanisms that activate low avidity cancer antigen-specific T cells. Therefore, we developed high and low avidity HER-2/neu-specific TCR transgenic mouse colonies specific for the same HER-2/neu epitope to define the tolerance mechanisms that specifically affect high versus low avidity tumor-specific T cells.
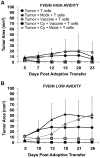
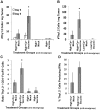
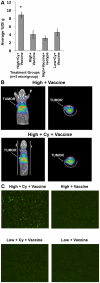
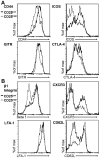
Methodology/principal findings: High and low avidity CD8(+) T cell receptor (TCR) transgenic mice specific for the breast cancer antigen HER-2/neu (neu) were developed to provide a purified source of naïve, tumor-specific T cells that can be used to study tolerance mechanisms. Adoptive transfer studies into tolerant FVB/N-derived HER-2/neu transgenic (neu-N) mice demonstrated that high avidity, but not low avidity, neu-specific T cells are inhibited by Tregs as the dominant tolerizing mechanism. High avidity T cells persisted, produced IFNγ, trafficked into tumors, and lysed tumors after adoptive transfer into mice treated with a neu-specific vaccine and low dose Cy to deplete Tregs. Analysis of Treg subsets revealed a Cy-sensitive CD4(+)Foxp3(+)CD25(low) tumor-seeking migratory phenotype, characteristic of effector/memory Tregs, and capable of high avidity T cell suppression.
Conclusion/significance: Depletion of CD25(low) Tregs allows activation of tumor-clearing high avidity T cells. Thus, the development of agents that specifically deplete Treg subsets should translate into more effective immunotherapies while avoiding autoimmunity.
Conflict of interest statement
Figures

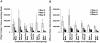

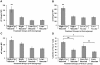


References
-
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. - PubMed
-
- Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, et al. Heterogeneous T-cell response to MAGE-A10(254–262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. - PubMed
-
- Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9:89–105. - PubMed
-
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. - PubMed
-
- Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nat Clin Pract Rheumatol. 2006;2:44–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

