Synthesis of functionalized dialkyl ketones from carboxylic acid derivatives and alkyl halides
- PMID: 22360350
- PMCID: PMC3308727
- DOI: 10.1021/ol300217x
Synthesis of functionalized dialkyl ketones from carboxylic acid derivatives and alkyl halides
Abstract
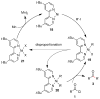
Unsymmetrical dialkyl ketones can be directly prepared by the nickel-catalyzed reductive coupling of carboxylic acid chlorides or (2-pyridyl)thioesters with alkyl iodides or benzylic chlorides. A wide variety of functional groups are tolerated by this process, including common nitrogen protecting groups and C-B bonds. Even hindered ketones flanked by tertiary and secondary centers can be formed. The mechanism is proposed to involve the reaction of a (L)Ni(alkyl)(2) intermediate with the carboxylic acid derivative.
Figures
References
-
- Dieter RK. Tetrahedron. 1999;55:4177.
- Lawrence JN. Perkin Trans. 1998;1:1739.
- Singh J, Satyamurthi N, Aidhen I. J Prakt Chem. 2000;342:340.
-
-
Ketones form stable linkages with hydrazide, hydroxylamino, and thiosemicarbazide groups under physiological conditions: Stephanopoulos N, Francis MB. Nat Chem Biol. 2011;7:876.Kalia J, Raines RT. Curr Org Chem. 2010;14:138.Sletten EM, Bertozzi CR. Angew Chem, Int Ed. 2009;48:6974.
-
-
-
An alternative strategy is the use of functionalized RMgX: Scheiper B, Bonnekessel M, Krause H, Fürstner A. J Org Chem. 2004;69:3943.
-
-
- Sato T, Naruse K, Enokiya M, Fujisawa T. Chem Lett. 1981;10:1135.
- Negishi E-i, Bagheri V, Chatterjee S, Luo F-T, Miller JA, Stoll AT. Tetrahedron Lett. 1983;24:5181.
- Tamaru Y, Ochiai H, Sanda F, Yoshida Z-i. Tetrahedron Lett. 1985;26:5529.
- Harada T, Kotani Y, Katsuhira T, Oku A. Tetrahedron Lett. 1991;32:1573.
- Asaoka M, Kosaka A, Tanaka M, Ueda T, Houkawa T, Takei H. Perkin Trans. 1997;1:2949.
- Wang D, Zhang Z. Org Lett. 2003;5:4645. - PubMed
- Yus M, Ortiz R. Eur J Org Chem. 2004;2004:3833.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources