Examination of glycan profiles from IgG-depleted human immunoglobulins facilitated by microscale affinity chromatography
- PMID: 22360417
- PMCID: PMC3320794
- DOI: 10.1021/ac203336u
Examination of glycan profiles from IgG-depleted human immunoglobulins facilitated by microscale affinity chromatography
Abstract
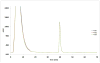
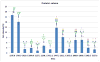
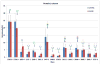
Among the most important proteins involved in disease and healing processes are the immunoglobulins (Igs). Although many of the Igs have been studied through proteomics, aside from IgG, immunoglobulin carbohydrates have not been extensively characterized in different states of health. It seems valuable to develop techniques that permit an understanding of changes in the structures and abundances of Ig glycans in the context of disease onset and progression. We have devised a strategy for characterization of the glycans for the Ig classes other than IgG (i.e., A, D, E, and M) that contain kappa light chains that requires only a few microliters of biological material. First, we designed a microcolumn containing recombinant Protein L that was immobilized on macroporous silica particles. A similarly designed Protein G microcolumn was utilized to first perform an online depletion of the IgG from the sample, human blood serum, and thereby facilitate enrichment of the other Igs. Even though only 3 μL of serum was used in these analyses, we were able to recover a significantly enriched fraction of non-IgG immunoglobulins. The enrichment properties of the Protein L column were characterized using a highly sensitive label-free quantitative proteomics LC-MS/MS approach, and the glycomic profiles of enriched immunoglobulins were measured by MALDI-TOF MS. As a proof of principle, a comparative study was conducted using blood serum from a small group of lung cancer patients and a group of age-matched cancer-free individuals to demonstrate that the method is suitable for investigation of glycosylation changes in disease. The results were in agreement with a glycomic investigation of whole blood serum from a much larger lung cancer cohort.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

