Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention
- PMID: 22360545
- PMCID: PMC3337338
- DOI: 10.3109/10409238.2012.661401
Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention
Abstract
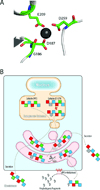
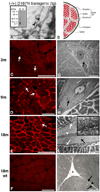
Protein misassembly into aggregate structures, including cross-β-sheet amyloid fibrils, is linked to diseases characterized by the degeneration of post-mitotic tissue. While amyloid fibril deposition in the extracellular space certainly disrupts cellular and tissue architecture late in the course of amyloid diseases, strong genetic, pathological and pharmacologic evidence suggests that the process of amyloid fibril formation itself, known as amyloidogenesis, likely causes these maladies. It seems that the formation of oligomeric aggregates during the amyloidogenesis process causes the proteotoxicity and cytotoxicity characteristic of these disorders. Herein, we review what is known about the genetics, biochemistry and pathology of familial amyloidosis of Finnish type (FAF) or gelsolin amyloidosis. Briefly, autosomal dominant D187N or D187Y mutations compromise Ca(2+) binding in domain 2 of gelsolin, allowing domain 2 to sample unfolded conformations. When domain 2 is unfolded, gelsolin is subject to aberrant furin endoproteolysis as it passes through the Golgi on its way to the extracellular space. The resulting C-terminal 68 kDa fragment (C68) is susceptible to extracellular endoproteolytic events, possibly mediated by a matrix metalloprotease, affording 8 and 5 kDa amyloidogenic fragments of gelsolin. These amyloidogenic fragments deposit systemically, causing a variety of symptoms including corneal lattice dystrophy and neurodegeneration. The first murine model of the disease recapitulates the aberrant processing of mutant plasma gelsolin, amyloid deposition, and the degenerative phenotype. We use what we have learned from our biochemical studies, as well as insight from mouse and human pathology to propose therapeutic strategies that may halt the progression of FAF.
Figures






References
-
- Ardalan MR, Shoja MM, Kiuru-Enari S. Amyloidosis-related nephrotic syndrome due to a G654A gelsolin mutation: the first report from the Middle East. Nephrol Dial Transplant. 2007;22:272–275. - PubMed
-
- Arumugam TV, Cheng Y-L, Choi Y, Choi Y-H, Yang S, Yun Y-K, Park J-S, Yang DK, Thundyil J, Gelderblom M, Karamyan VT, Tang S-C, Chan SL, Magnus T, Sobey CG, Jo D-G. Evidence that γ-secretase-mediated Notch signaling induces neuronal cell death via the nuclear factor-κB-Bcl-2-interacting mediator of cell death pathway in ischemic stroke. Mol Pharmacol. 2011;80:23–31. - PubMed
-
- Ashish, Paine MS, Perryman PB, Yang L, Yin HL, Krueger JK. Global structure changes associated with Ca2+ activation of full-length human plasma gelsolin. J Biol Chem. 2007;282:25884–25892. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Baures PW, Oza VB, Peterson SA, Kelly JW. Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the nonsteroidal antiinflammatory drug flufenamic acid. Bioorg Med Chem. 1999;7:1339–1347. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
