Sequential allylic C-H amination/vinylic C-H arylation: a strategy for unnatural amino acid synthesis from α-olefins
- PMID: 22360547
- PMCID: PMC4104998
- DOI: 10.1021/ol300063t
Sequential allylic C-H amination/vinylic C-H arylation: a strategy for unnatural amino acid synthesis from α-olefins
Abstract
Tandem reaction sequences that selectively convert multiple C-H bonds of abundant hydrocarbon feedstocks to functionalized materials enable rapid buildup of molecular complexity in an economical way. A tandem C-H amination/vinylic C-H arylation reaction sequence is described under Pd(II)/sulfoxide-catalysis that furnishes a wide range of α- and β-homophenylalanine precursors from commodity α-olefins and readily available aryl boronic acids. General routes to enantiopure amino acid esters and densely functionalized homophenylalanine derivatives are demonstrated.
Figures


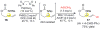


References
-
-
General C—H oxidation, aminations, alkylations: Müller P, Fruit C. Chem Rev. 2003;103:2905.Espino CG, DuBois J. In: Modern Rhodium-Catalyzed Organic Reactions. Evans PA, editor. Wiley-VCH; Weinheim; 2005. p. 379.Huard K, Lebel H. Chem Eur J. 2008;14:6222.Dick AR, Sanford MS. Tetrahedron. 2006;62:2439. and references therein.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174.Chen MS, White MC. Science. 2007;318:783.Davies HML, Manning JR. Nature. 2008;451:417.Lewis JC, Bergman RG, Ellman JA. Acc Chem Res. 2008;41:1013.Lafrance M, Lapointe D, Fagnou K. Tetrahedron. 2008;64:6015.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem Int Ed. 2009;48:5094.Phipps RJ, Gaunt MJ. Science. 2009;323:1593.Chen MS, White MC. Science. 2010;327:566.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654.Das S, Incarvito CD, Crabtree RH, Brudvig G. Science. 2006;312:1941.Dobereiner GE, Crabtree RH. Chem Rev. 2010;110:681.Paradine SM, White MC. J Am Chem Soc. 2012;134:2036.
-
-
-
Pd-catalyzed allylic C—H oxidations: Chen MS, White MC. J Am Chem Soc. 2004;126:1346.Chen MS, Prabagaran N, Labenz NA, White MC. J Am Chem Soc. 2005;127:6970.Fraunhoffer KJ, Prabagaran N, Sirois LE, White MC. J Am Chem Soc. 2006;128:9032.Delcamp JH, White MC. J Am Chem Soc. 2006;128:15076.Covell DJ, White MC. Angew Chem Int Ed. 2008;47:6448.Vermeulen NA, Delcamp JH, White MC. J Am Chem Soc. 2010;132:11323.Stang EM, White MC. Nat Chem. 2009;1:547.Stang EM, White MC. Angew Chem Int Ed. 2011;50:2094.Mitsudome T, Umetani T, Nosaka N, Mori K, Mizugaki T, Ebitani K, Kaneda K. Angew Chem Int Ed. 2006;45:481.Pilarski LT, Selander N, Bose D, Szabo KJ. Org Lett. 2009;11:5518.Lin BL, Labinger JA, Bercaw JE. Can J Chem. 2009;87:264.Thiery E, Aouf C, Belloy J, Harakat D, Le Bras J, Muzart J. J Org Chem. 2010;75:1771.Henderson WH, Check CT, Proust N, Stambuli JP. Org Lett. 2010;12:824.
-
-
-
Pd-catalyzed allylic C—H aminations: Larock RC, Hightower TR, Hasvold LA, Peterson KP. J Org Chem. 1996;61:3584.Fraunhoffer KJ, White MC. J Am Chem Soc. 2007;129:7274.Reed SA, White MC. J Am Chem Soc. 2008;130:3316.Liu G, Yin G, Wu L. Angew Chem Int Ed. 2008;47:4733.Rice GT, White MC. J Am Chem Soc. 2009;131:11707.Reed SA, Mazzotti AR, White MC. J Am Chem Soc. 2009;131:11701.Qi X, Rice GT, Lall MS, Plummer MS, White MC. Tetrahedron. 2010;66:4816.Nahra F, Liron F, Prestat G, Mealli C, Messaoudi A, Poli G. Chem Eur J. 2009;15:11078.Shimizu Y, Obora Y, Ishii Y. Org Lett. 2010;12:1372.
-
-
-
Pd-catalyzed allylic C—H alkylation: Young AJ, White MC. J Am Chem Soc. 2008;130:14090.Young AJ, White MC. Angew Chem Int Ed. 2011;50:6824.Lin S, Song CX, Cai GX, Wang WH, Shi ZJ. J Am Chem Soc. 2008;130:12901.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

