Structural, EPR, and Mössbauer characterization of (μ-alkoxo)(μ-carboxylato)diiron(II,III) model complexes for the active sites of mixed-valent diiron enzymes
- PMID: 22360600
- PMCID: PMC3298377
- DOI: 10.1021/ic2021726
Structural, EPR, and Mössbauer characterization of (μ-alkoxo)(μ-carboxylato)diiron(II,III) model complexes for the active sites of mixed-valent diiron enzymes
Abstract
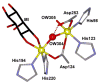
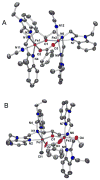
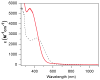
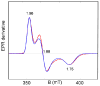
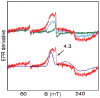
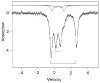
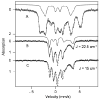
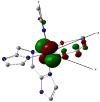
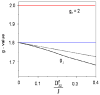
To obtain structural and spectroscopic models for the diiron(II,III) centers in the active sites of diiron enzymes, the (μ-alkoxo)(μ-carboxylato)diiron(II,III) complexes [Fe(II)Fe(III)(N-Et-HPTB)(O(2)CPh)(NCCH(3))(2)](ClO(4))(3) (1) and [Fe(II)Fe(III)(N-Et-HPTB)(O(2)CPh)(Cl)(HOCH(3))](ClO(4))(2) (2) (N-Et-HPTB = N,N,N',N'-tetrakis(2-(1-ethyl-benzimidazolylmethyl))-2-hydroxy-1,3-diaminopropane) have been prepared and characterized by X-ray crystallography, UV-visible absorption, EPR, and Mössbauer spectroscopies. Fe1-Fe2 separations are 3.60 and 3.63 Å, and Fe1-O1-Fe2 bond angles are 128.0° and 129.4° for 1 and 2, respectively. Mössbauer and EPR studies of 1 show that the Fe(III) (S(A) = 5/2) and Fe(II) (S(B) = 2) sites are antiferromagnetically coupled to yield a ground state with S = 1/2 (g= 1.75, 1.88, 1.96); Mössbauer analysis of solid 1 yields J = 22.5 ± 2 cm(-1) for the exchange coupling constant (H = JS(A)·S(B) convention). In addition to the S = 1/2 ground-state spectrum of 1, the EPR signal for the S = 3/2 excited state of the spin ladder can also be observed, the first time such a signal has been detected for an antiferromagnetically coupled diiron(II,III) complex. The anisotropy of the (57)Fe magnetic hyperfine interactions at the Fe(III) site is larger than normally observed in mononuclear complexes and arises from admixing S > 1/2 excited states into the S = 1/2 ground state by zero-field splittings at the two Fe sites. Analysis of the "D/J" mixing has allowed us to extract the zero-field splitting parameters, local g values, and magnetic hyperfine structural parameters for the individual Fe sites. The methodology developed and followed in this analysis is presented in detail. The spin Hamiltonian parameters of 1 are related to the molecular structure with the help of DFT calculations. Contrary to what was assumed in previous studies, our analysis demonstrates that the deviations of the g values from the free electron value (g = 2) for the antiferromagnetically coupled diiron(II,III) core in complex 1 are predominantly determined by the anisotropy of the effective g values of the ferrous ion and only to a lesser extent by the admixture of excited states into ground-state ZFS terms (D/J mixing). The results for 1 are discussed in the context of the data available for diiron(II,III) clusters in proteins and synthetic diiron(II,III) complexes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

