From basic to clinical neuropharmacology: targetophilia or pharmacodynamics?
- PMID: 22360689
- PMCID: PMC3391528
- DOI: 10.1111/j.1365-2125.2012.04246.x
From basic to clinical neuropharmacology: targetophilia or pharmacodynamics?
Abstract
Historically, much drug discovery and development in psychopharmacology tended to be empirical. However, over the last 20 years it has primarily been target oriented, with synthesis and selection of compounds designed to act at a specific neurochemical site. Such compounds are then examined in functional animal models of disease. There is little evidence that this approach (which we call 'targetophilia') has enhanced the discovery process and some indications that it may have retarded it. A major problem is the weakness of many animal models in mimicking the disease and the lack of appropriate biochemical markers of drug action in animals and patients. In this review we argue that preclinical studies should be conducted as if they were clinical studies in design, analysis, and reporting, and that clinical pharmacologists should be involved at the earliest stages, to help ensure that animal models reflect as closely as possible the clinical disease. In addition, their familiarity with pharmacokinetic-pharmacodynamic integration (PK-PD) would help ensure that appropriate dosing and drug measurement techniques are applied to the discovery process, thereby producing results with relevance to therapeutics. Better integration of experimental and clinical pharmacologists early in the discovery process would allow observations in animals and patients to be quickly exchanged between the two disciplines. This non-linear approach to discovery used to be the way research proceeded, and it resulted in productivity that has never been bettered. It also follows that occasionally 'look-see' studies, a proven technique for drug discovery, deserve to be reintroduced.
© 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
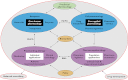
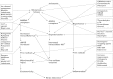
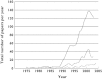
References
-
- Bush V. Science – the Endless Frontier. A Report to the President on a Program for Postwar Scientific Research. Washington, DC: Office of Scientific Research and Development; 1945.
-
- Stokes DE. Pasteur's Quadrant. Basic Science and Technological Innovation. Washington, DC: Brookings Institution Press; 1997.
-
- Ostrowski J, Wyrwicz LS. Integrating genomics, proteomics and bioinformatics in translational studies of molecular medicine. Expert Rev Mol Diagn. 2009;9:623–30. - PubMed
-
- Noble D. The Music of Life. Biology Beyond the Genome. Oxford: Oxford University Press; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

