Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice
- PMID: 22360721
- PMCID: PMC4323161
- DOI: 10.1042/BJ20112197
Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice
Abstract
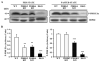
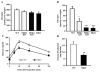
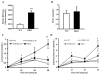
The importance of PDHK (pyruvate dehydrogenase kinase) 2 and 4 in regulation of the PDH complex (pyruvate dehydrogenase complex) was assessed in single- and double-knockout mice. PDHK2 deficiency caused higher PDH complex activity and lower blood glucose levels in the fed, but not the fasted, state. PDHK4 deficiency caused similar effects, but only after fasting. Double deficiency intensified these effects in both the fed and fasted states. PDHK2 deficiency had no effect on glucose tolerance, PDHK4 deficiency produced only a modest effect, but double deficiency caused a marked improvement and also induced lower insulin levels and increased insulin sensitivity. In spite of these beneficial effects, the double-knockout mice were more sensitive than wild-type and single-knockout mice to long-term fasting, succumbing to hypoglycaemia, ketoacidosis and hypothermia. Stable isotope flux analysis indicated that hypoglycaemia was due to a reduced rate of gluconeogenesis and that slightly more glucose was converted into ketone bodies in the double-knockout mice. The findings establish that PDHK2 is more important in the fed state, PDHK4 is more important in the fasted state, and survival during long-term fasting depends upon regulation of the PDH complex by both PDHK2 and PDHK4.
Figures

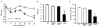

Similar articles
-
Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation.Biochem J. 2006 Aug 1;397(3):417-25. doi: 10.1042/BJ20060125. Biochem J. 2006. PMID: 16606348 Free PMC article.
-
Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet.Biochem J. 2009 Sep 25;423(2):243-52. doi: 10.1042/BJ20090390. Biochem J. 2009. PMID: 19627255
-
FoxO1 regulates myocardial glucose oxidation rates via transcriptional control of pyruvate dehydrogenase kinase 4 expression.Am J Physiol Heart Circ Physiol. 2017 Sep 1;313(3):H479-H490. doi: 10.1152/ajpheart.00191.2017. Epub 2017 Jul 7. Am J Physiol Heart Circ Physiol. 2017. PMID: 28687587
-
AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats.Biochem Soc Trans. 2003 Dec;31(Pt 6):1165-7. doi: 10.1042/bst0311165. Biochem Soc Trans. 2003. PMID: 14641018 Review.
-
Microenvironmental control of glucose metabolism in tumors by regulation of pyruvate dehydrogenase.Int J Cancer. 2019 Feb 15;144(4):674-686. doi: 10.1002/ijc.31812. Epub 2018 Oct 4. Int J Cancer. 2019. PMID: 30121950 Free PMC article. Review.
Cited by
-
Real-time hyperpolarized 13C magnetic resonance detects increased pyruvate oxidation in pyruvate dehydrogenase kinase 2/4-double knockout mouse livers.Sci Rep. 2019 Nov 11;9(1):16480. doi: 10.1038/s41598-019-52952-6. Sci Rep. 2019. PMID: 31712597 Free PMC article.
-
Analysis of a genetic region affecting mouse body weight.Physiol Genomics. 2023 Mar 1;55(3):132-146. doi: 10.1152/physiolgenomics.00137.2022. Epub 2023 Jan 30. Physiol Genomics. 2023. PMID: 36717164 Free PMC article.
-
Ketone body metabolism and cardiovascular disease.Am J Physiol Heart Circ Physiol. 2013 Apr 15;304(8):H1060-76. doi: 10.1152/ajpheart.00646.2012. Epub 2013 Feb 8. Am J Physiol Heart Circ Physiol. 2013. PMID: 23396451 Free PMC article. Review.
-
Structure-guided development of specific pyruvate dehydrogenase kinase inhibitors targeting the ATP-binding pocket.J Biol Chem. 2014 Feb 14;289(7):4432-43. doi: 10.1074/jbc.M113.533885. Epub 2013 Dec 19. J Biol Chem. 2014. PMID: 24356970 Free PMC article.
-
Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease.Front Physiol. 2014 Jul 17;5:260. doi: 10.3389/fphys.2014.00260. eCollection 2014. Front Physiol. 2014. PMID: 25101001 Free PMC article. Review.
References
-
- Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–259. - PubMed
-
- Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. - PubMed
-
- Popov KM, Kedishvili NY, Zhao Y, Gudi R, Harris RA. Molecular cloning of the p45 subunit of pyruvate dehydrogenase kinase. J Biol Chem. 1994;269:29720–29724. - PubMed
-
- Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–28994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

