Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials
- PMID: 22360996
- PMCID: PMC3328963
- DOI: 10.1161/CIRCIMAGING.111.969923
Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials
Abstract
Background: An increase in left ventricular mass (LVM) is associated with mortality and cardiovascular morbidity in patients with end-stage renal disease.
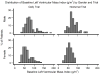
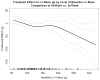
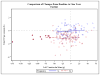
Methods and results: The Frequent Hemodialysis Network (FHN) Daily Trial randomized 245 patients to 12 months of 6 times per week daily in-center hemodialysis or conventional hemodialysis; the FHN Nocturnal Trial randomized 87 patients to 12 months of 6 times per week nocturnal hemodialysis or conventional hemodialysis. The main cardiac secondary outcome was change in LVM. In each trial, we examined whether several predefined baseline demographic or clinical factors as well as change in volume removal, blood pressure, or solute clearance influenced the effect of frequent hemodialysis on LVM. In the Daily Trial, frequent hemodialysis resulted in a significant reduction in LVM (13.1 g; 95% CI, 5.0-21.3 g; P=0.002), LVM index (6.9 g/m(2); 95% CI, 2.4-11.3 g/m(2); P=0.003), and percent change in geometric mean of LVM (7.0%; 95% CI, 1.0%-12.6; P=0.02). Similar trends were noted in the Nocturnal Trial but did not reach statistical significance. In the Daily Trial, a more pronounced effect of frequent hemodialysis on LVM was evident among patients with left ventricular hypertrophy at baseline. Changes in LVM were associated with changes in blood pressure (conventional hemodialysis: R=0.28, P=0.01, daily hemodialysis: R=0.54, P<0.001) and were not significantly associated with changes in other parameters.
Conclusions: Frequent in-center hemodialysis reduces LVM. The benefit of frequent hemodialysis on LVM may be mediated by salutary effects on blood pressure. Clinical Trial Registration- URL: http://www.clinicaltrials.gov. Unique identifier: NCT00264758.
Figures






References
-
- Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. - PubMed
-
- Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–1498. - PubMed
-
- London GM. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial. 2003;16:85–94. - PubMed
-
- Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial. 2003;16:111–117. - PubMed
-
- Zoccali C, Mallamaci F, Tripepi G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int Suppl. 2003:105–110. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

