An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages
- PMID: 22361007
- PMCID: PMC3315380
- DOI: 10.1016/j.immuni.2012.02.001
An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages
Abstract
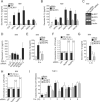
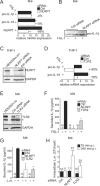
Cytosolic pathogen- and damage-associated molecular patterns are sensed by pattern recognition receptors, including members of the nucleotide-binding domain and leucine-rich repeat-containing gene family (NLR), which cause inflammasome assembly and caspase-1 activation to promote maturation and release of the inflammatory cytokines interleukin-1β (IL-1β) and IL-18 and induction of pyroptosis. However, the contribution of most of the NLRs to innate immunity, host defense, and inflammasome activation and their specific agonists are still unknown. Here we describe identification and characterization of an NLRP7 inflammasome in human macrophages, which is induced in response to microbial acylated lipopeptides. Activation of NLRP7 promoted ASC-dependent caspase-1 activation, IL-1β and IL-18 maturation, and restriction of intracellular bacterial replication, but not caspase-1-independent secretion of the proinflammatory cytokines IL-6 and tumor necrosis factor-α. Our study therefore increases our currently limited understanding of NLR activation, inflammasome assembly, and maturation of IL-1β and IL-18 in human macrophages.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




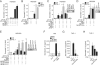
Comment in
-
Innate immunity: Linking mitochondria and microbes to inflammasomes.Nat Rev Immunol. 2012 Mar 9;12(4):229. doi: 10.1038/nri3195. Nat Rev Immunol. 2012. PMID: 22402669 No abstract available.
References
-
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. - PubMed
-
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

