Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis
- PMID: 22361023
- PMCID: PMC3334411
- DOI: 10.1016/j.expneurol.2012.02.002
Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis
Abstract
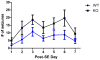
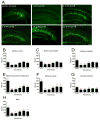
Recent evidence suggests that astrocytes may be a potential new target for the treatment of epilepsy. The glial water channel aquaporin-4 (AQP4) is expressed in astrocytes, and along with the inwardly-rectifying K(+) channel K(ir)4.1 is thought to underlie the reuptake of H(2)O and K(+) into glial cells during neural activity. Previous studies have demonstrated increased seizure duration and slowed potassium kinetics in AQP4(-/-) mice, and redistribution of AQP4 in hippocampal specimens from patients with chronic epilepsy. However, the regulation and role of AQP4 during epileptogenesis remain to be defined. In this study, we examined the expression of AQP4 and other glial molecules (GFAP, K(ir)4.1, glutamine synthetase) in the intrahippocampal kainic acid (KA) model of epilepsy and compared behavioral and histologic outcomes in wild-type mice vs. AQP4(-/-) mice. Marked and prolonged reduction in AQP4 immunoreactivity on both astrocytic fine processes and endfeet was observed following KA status epilepticus in multiple hippocampal layers. In addition, AQP4(-/-) mice had more spontaneous recurrent seizures than wild-type mice during the first week after KA SE as assessed by chronic video-EEG monitoring and blinded EEG analysis. While both genotypes exhibited similar reactive astrocytic changes, granule cell dispersion and CA1 pyramidal neuron loss, there were an increased number of fluorojade-positive cells early after KA SE in AQP4(-/-) mice. These results indicate a marked reduction of AQP4 following KA SE and suggest that dysregulation of water and potassium homeostasis occurs during early epileptogenesis. Restoration of astrocytic water and ion homeostasis may represent a novel therapeutic strategy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures






References
-
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proceedings of the National Academy of Sciences. 2003;100:13615–13620. - PMC - PubMed
-
- Andrew RD, Fagan M, Ballyk BA, Rosen AS. Seizure susceptibility and the osmotic state. Brain Res. 1989;498:175–180. - PubMed
-
- Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM. Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Experimental Neurology. 2005;194:76–90. - PubMed
-
- Binder DK, Oshio K, Ma T, Verkman AS, Manley GT. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport. 2004;15:259–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

