Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results
- PMID: 22361316
- PMCID: PMC3343212
- DOI: 10.1016/j.ophtha.2011.11.030
Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results
Abstract
Objective: The purpose of this study was to identify possible risk factors for retroprosthetic membrane (RPM) development in a large, multicenter cohort of patients receiving a Boston type 1 keratoprosthesis.
Design: Cohort study.
Participants: The final analysis included 265 eyes of 265 patients who underwent implantation of a Boston keratoprosthesis type I device between January 2003 and July 2008 by 1 of 19 surgeons at 18 medical centers.
Methods: Forms reporting preoperative, intraoperative, and postoperative parameters were prospectively collected and subsequently analyzed at a central data collection site.
Main outcome measures: The primary outcome was the presence or absence of an RPM during the follow-up period.
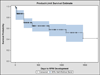
Results: The average age of patients was 63.3±19.1 years, 48.5% of the patients were female, and 52.5% of procedures were performed on the right eye. The mean follow-up time was 17.8±14.9 months. The majority (85.4%; n = 222) had undergone an average of 2.2±1.2 (range, 1-8) penetrating keratoplasties before keratoprosthesis implantation, and 38 eyes (14.6%) received a primary keratoprosthesis. The overall RPM formation rate was 31.7% (n = 84). The most significant risk factor for RPM development was infectious keratitis (as a surgical indication for keratoprosthesis surgery itself), resulting in a rate of RPM formation of 70.6%. As an independent risk factor, the hazard ratio (HR) of RPM development in these eyes was 3.20 (95% confidence interval, 1.66-6.17). Aniridia was also an independent risk factor for RPM development (HR, 3.13; 95% confidence interval, 1.10-8.89).
Conclusions: Formation of RPM is a common complication of keratoprosthesis surgery, occurring in approximately one-third of cases. Eyes at the highest risk of RPM development are those receiving corneal replacement for infectious keratitis and aniridia.
Copyright © 2012 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All of the authors have no significant conflict to report.
Figures

References
-
- Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116:640–651. - PubMed
-
- Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: the University of California Davis experience. Cornea. 2009;28:321–327. - PubMed
-
- Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996. - PubMed
-
- Zerbe BL, Belin MW, Ciolino JB, Boston Type 1 Keratoprosthesis Study Group Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779–1784. - PubMed
-
- Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751–757. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

