Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting
- PMID: 22361433
- PMCID: PMC3314175
- DOI: 10.18632/aging.100436
Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting
Abstract
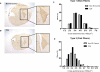
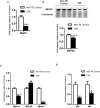
Cancer cachexia is a highly debilitating paraneoplastic disease observed in more than 50% of patients with advanced cancers and directly contributes to 20% of cancer deaths. Loss of skeletal muscle is a defining characteristic of patients with cancer cachexia and is associated with poor survival. The present study reveals the involvement of a myogenic transcription factor Myocyte Enhancer Factor (MEF) 2C in cancer-induced skeletal muscle wasting. Increased skeletal muscle mRNA expression of Suppressor of Cytokine Signaling (Socs) 3 and the IL-6 receptor indicative of active IL-6 signaling was seen in skeletal muscle of mice bearing the Colon 26 (C26) carcinoma. Loss of skeletal muscle structural integrity and distorted mitochondria were also observed using electron microscopy. Gene and protein expression of MEF2C was significantly downregulated in skeletal muscle from C26-bearing mice. MEF2C gene targets myozenin and myoglobin as well as myokinase were also altered during cachexia, suggesting dysregulated oxygen transport capacity and ATP regeneration in addition to distorted structural integrity. In addition, reduced expression of calcineurin was observed which suggested a potential pathway of MEF2C dysregulation. Together, these effects may limit sarcomeric contractile ability and also predispose skeletal muscle to structural instability; associated with muscle wasting and fatigue in cachexia.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures



Similar articles
-
MEF2c-Dependent Downregulation of Myocilin Mediates Cancer-Induced Muscle Wasting and Associates with Cachexia in Patients with Cancer.Cancer Res. 2020 May 1;80(9):1861-1874. doi: 10.1158/0008-5472.CAN-19-1558. Epub 2020 Mar 4. Cancer Res. 2020. PMID: 32132110 Free PMC article.
-
Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C101-15. doi: 10.1152/ajpcell.00344.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122162
-
JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia.Am J Physiol Endocrinol Metab. 2012 Aug 1;303(3):E410-21. doi: 10.1152/ajpendo.00039.2012. Epub 2012 Jun 5. Am J Physiol Endocrinol Metab. 2012. PMID: 22669242 Free PMC article.
-
Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation.Am J Physiol Regul Integr Comp Physiol. 2020 Feb 1;318(2):R296-R310. doi: 10.1152/ajpregu.00147.2019. Epub 2019 Dec 11. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31823669 Review.
-
Endoplasmic Reticulum Stress, Calcium Dysregulation and Altered Protein Translation: Intersection of Processes That Contribute to Cancer Cachexia Induced Skeletal Muscle Wasting.Curr Drug Targets. 2016;17(10):1140-6. doi: 10.2174/1389450116666150416115721. Curr Drug Targets. 2016. PMID: 25882219 Review.
Cited by
-
Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs.Oncotarget. 2016 Jul 12;7(28):43442-43460. doi: 10.18632/oncotarget.9779. Oncotarget. 2016. PMID: 27259276 Free PMC article.
-
Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction.J Cachexia Sarcopenia Muscle. 2020 Feb;11(1):226-240. doi: 10.1002/jcsm.12498. Epub 2019 Nov 13. J Cachexia Sarcopenia Muscle. 2020. PMID: 31965747 Free PMC article.
-
Myocyte-specific enhancer factor 2D promotes tumorigenesis and progression in tongue squamous cell carcinoma.Int J Clin Exp Pathol. 2020 May 1;13(5):934-943. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 32509064 Free PMC article.
-
Skeletal muscle wasting after a severe burn is a consequence of cachexia and sarcopenia.JPEN J Parenter Enteral Nutr. 2021 Nov;45(8):1627-1633. doi: 10.1002/jpen.2238. Epub 2021 Sep 2. JPEN J Parenter Enteral Nutr. 2021. PMID: 34296448 Free PMC article. Review.
-
A Metabolic Change towards Fermentation Drives Cancer Cachexia in Myotubes.Biomedicines. 2021 Jun 20;9(6):698. doi: 10.3390/biomedicines9060698. Biomedicines. 2021. PMID: 34203023 Free PMC article.
References
-
- Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 2005;20:340–348. - PubMed
-
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. - PubMed
-
- Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. - PubMed
-
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

