Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo
- PMID: 22361619
- PMCID: PMC3405840
- DOI: 10.4161/auto.19048
Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo
Abstract
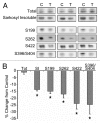
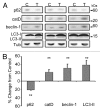
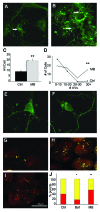
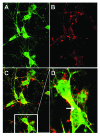
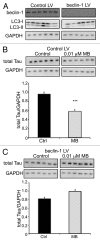
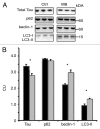
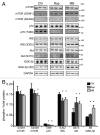
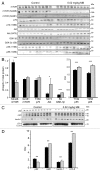
More than 30 neurodegenerative diseases including Alzheimer disease (AD), frontotemporal lobe dementia (FTD), and some forms of Parkinson disease (PD) are characterized by the accumulation of an aggregated form of the microtubule-binding protein tau in neurites and as intracellular lesions called neurofibrillary tangles. Diseases with abnormal tau as part of the pathology are collectively known as the tauopathies. Methylthioninium chloride, also known as methylene blue (MB), has been shown to reduce tau levels in vitro and in vivo and several different mechanisms of action have been proposed. Herein we demonstrate that autophagy is a novel mechanism by which MB can reduce tau levels. Incubation with nanomolar concentrations of MB was sufficient to significantly reduce levels of tau both in organotypic brain slice cultures from a mouse model of FTD, and in cell models. Concomitantly, MB treatment altered the levels of LC3-II, cathepsin D, BECN1, and p62 suggesting that it was a potent inducer of autophagy. Further analysis of the signaling pathways induced by MB suggested a mode of action similar to rapamycin. Results were recapitulated in a transgenic mouse model of tauopathy administered MB orally at three different doses for two weeks. These data support the use of this drug as a therapeutic agent in neurodegenerative diseases.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
