Esophageal functional impairments in experimental eosinophilic esophagitis
- PMID: 22361731
- PMCID: PMC3378164
- DOI: 10.1152/ajpgi.00013.2012
Esophageal functional impairments in experimental eosinophilic esophagitis
Abstract
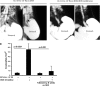
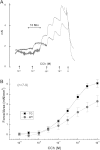
Eosinophilic esophagitis (EoE) is an emerging chronic esophageal disease. Despite the increasing diagnosis of EoE globally, the causes of EoE and other esophageal eosinophilic disorders are not clearly understood. EoE pathology includes accumulation of inflammatory cells (e.g., eosinophils, mast cells), characteristic endoscopic features (e.g., furrows, the formation of fine concentric mucosal rings, exudates), and functional impairments (e.g., esophageal stricture, dysmotility). We hypothesized that the esophageal structural pathology and functional impairments of EoE develop as a consequence of the effector functions of the accumulated inflammatory cells. We analyzed eosinophils (anti-major basic protein immunostaining), esophageal stricture (X-ray barium swallowing), and esophageal motility (isometric force) in two established transgenic murine models of EoE (CD2-IL-5 and rtTA-CC10-IL-13) and a novel eosinophil-deficient model (ΔdblGATA/CD2-IL-5). Herein, we show the following: 1) CD2-IL-5 and doxycycline (DOX)-induced rtTA-CC10-IL-13 mice have chronic eosinophilic and mast cell esophageal inflammation; 2) eosinophilic esophageal inflammation promotes esophageal stricture in both transgenic murine models; 3) the eosinophil-deficient ΔdblGATA/CD-2-IL-5 mice were protected from the induction of stricture, whereas the eosinophil-competent CD2-IL-5 mice develop esophageal stricture; 4) esophageal stricture is not reversible in DOX-induced rtTA-CC10-IL-13 mice (8 wk DOX followed by 8 wk no-DOX); and 5) IL-5 transgene-induced (CD2-IL-5) EoE evidences esophageal dysmotility (relaxation and contraction) that is independent of the eosinophilic esophageal inflammation: CD2-IL-5 and ΔdblGATA/CD2-IL-5 mice have comparable esophageal dysmotility. Collectively, our present study directly implicates chronic eosinophilic inflammation in the development of the esophageal structural impairments of experimental EoE.
Figures





References
-
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 38: 109–116, 1993 - PubMed
-
- Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116: 536–547, 2006 - PMC - PubMed
-
- Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis: It's not just kid's stuff. Gastrointest Endosc 56: 260–270, 2002 - PubMed
-
- Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133: 1342–1363, 2007 - PubMed
-
- Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 39: 177–253, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

