Occludin is required for apoptosis when claudin-claudin interactions are disrupted
- PMID: 22361748
- PMCID: PMC3288343
- DOI: 10.1038/cddis.2012.14
Occludin is required for apoptosis when claudin-claudin interactions are disrupted
Abstract
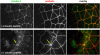
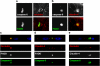
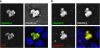
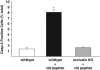
Disruption of tight junctions is often seen during pathogen infection, inflammation, and tumor progression. Mislocalization of the tight junction proteins occludin and claudin in mammary epithelial monolayers leads to apoptosis through the extrinsic pathway. To further investigate the mechanism of this response, a normal mammary epithelial cell line (EpH4) as well as primary mammary epithelial cells were treated with a claudin-disrupting mimic peptide, DFYNP (aspartic acid-phenylalanine-tyrosine-asparagine-proline). Using fluorescent indicators, we found that caspase-3 activation, resulting from treatment with DFYNP, was restricted to EpH4 and primary mammary epithelial cells with mislocalized claudin-4. Mislocalized claudin-4 and occludin were colocalized in non-junctional puncta, and both molecules were found in the death-inducing signaling complex (DISC) where they colocalized with Fas, fas-associated protein with death domain (FADD), active caspase-8 and caspase-3 at distinct apical domains. Importantly, caspase-3 activation was totally repressed in primary mammary epithelial cells from occludin null mice. Thus, the apoptotic response appears to be initiated by the movement of occludin to the DISC suggesting that this molecule has signaling properties that initiate cell death when its tight junction location is disrupted.
Figures



References
-
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. - PubMed
-
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. - PubMed
-
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. - PubMed
-
- Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

