Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization
- PMID: 22362173
- PMCID: PMC3314353
- DOI: 10.2337/db10-1492
Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization
Abstract
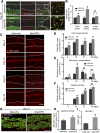
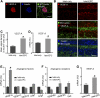
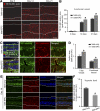
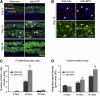
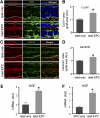
Impaired revascularization of transplanted islets is a critical problem that leads to progressive islet loss. Since endothelial progenitor cells (EPCs) are known to aid neovascularization, we aimed to enhance islet engraftment by cotransplanting EPCs with islets. Porcine islets, with (islet-EPC group) or without (islet-only group) human cord blood-derived EPCs, were transplanted into diabetic nude mice. The islet-EPC group reached euglycemia by ∼11 days posttransplantation, whereas the islet-only group did not. Also, the islet-EPC group had a higher serum porcine insulin level than the islet-only group. Islets from the islet-EPC group were more rapidly revascularized at the early period of transplantation without increment of final capillary density at the fully revascularized graft. Enhanced revascularization rate in the islet-EPC group was mainly attributed to stimulating vascular endothelial growth factor-A production from the graft. The rapid revascularization by EPC cotransplantation led to better graft perfusion and recovery from hypoxia. EPC cotransplantation was also associated with greater β-cell proliferation, probably by more basement membrane production and hepatocyte growth factor secretion. In conclusion, cotransplantation of EPCs and islets induces better islet engraftment by enhancing the rate of graft revascularization. These findings might provide a directly applicable tool to enhance the efficacy of islet transplantation in clinical practice.
Figures

References
-
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567 - PubMed
-
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med 2003;9:661–668 - PubMed
-
- Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 2006;10:397–405 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

