Preexisting autoantibodies predict efficacy of oral insulin to cure autoimmune diabetes in combination with anti-CD3
- PMID: 22362174
- PMCID: PMC3357270
- DOI: 10.2337/db11-1304
Preexisting autoantibodies predict efficacy of oral insulin to cure autoimmune diabetes in combination with anti-CD3
Abstract
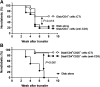
We have previously developed a combination therapy (CT) using anti-CD3 monoclonal antibodies together with islet-(auto)antigen immunizations that can more efficiently reverse type 1 diabetes (T1D) than either entity alone. However, clinical translation of antigen-specific therapies in general is hampered by the lack of biomarkers that could be used to optimize the modalities of antigen delivery and to predict responders from nonresponders. To support the rapid identification of candidate biomarkers, we systematically evaluated multiple variables in a mathematical disease model. The in silico predictions were validated by subsequent laboratory data in NOD mice with T1D that received anti-CD3/oral insulin CT. Our study shows that higher anti-insulin autoantibody levels at diagnosis can distinguish responders and nonresponders among recipients of CT exquisitely well. In addition, early posttreatment changes in proinflammatory cytokines were indicative of long-term remission. Coadministration of oral insulin improved and prolonged the therapeutic efficacy of anti-CD3 therapy, and long-term protection was achieved by maintaining elevated insulin-specific regulatory T cell numbers that efficiently lowered diabetogenic effector memory T cells. Our validation of preexisting autoantibodies as biomarkers to distinguish future responders from nonresponders among recipients of oral insulin provides a compelling and mechanistic rationale to more rapidly translate anti-CD3/oral insulin CT for human T1D.
Figures




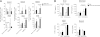
References
-
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 - PubMed
-
- Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 - PubMed
-
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

