Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet
- PMID: 22362175
- PMCID: PMC3357272
- DOI: 10.2337/db11-1498
Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet
Abstract
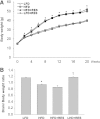
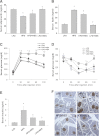
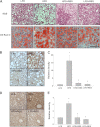
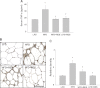
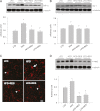
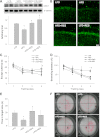
Obesity-induced diabetes is associated with chronic inflammation and is considered a risk factor for neurodegeneration. We tested the hypothesis that an AMP-activated protein kinase activator, resveratrol (RES), which is known to exert potent anti-inflammatory effects, would attenuate peripheral and central inflammation and improve memory deficit in mice fed a high-fat diet (HFD). C57BL/6J mice were fed an HFD or an HFD supplemented with RES for 20 weeks. Metabolic parameters in serum were evaluated, and Western blot analysis and immunohistochemistry in peripheral organs and brain were completed. We used the Morris water maze test to study the role of RES on memory function in HFD-treated mice. RES treatment reduced hepatic steatosis, macrophage infiltration, and insulin resistance in HFD-fed mice. In the hippocampus of HFD-fed mice, the protein levels of tumor necrosis factor-α and Iba-1 expression were reduced by RES treatment. Choline acetyltransferase was increased, and the phosphorylation of tau was decreased in the hippocampus of HFD-fed mice upon RES treatment. In particular, we found that RES significantly improved memory deficit in HFD-fed mice. These findings indicate that RES reverses obesity-related peripheral and central inflammation and metabolic derangements and improves memory deficit in HFD-fed diabetic mice.
Figures


References
-
- Muhammad S, Bierhaus A, Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer’s disease. J Alzheimers Dis 2009;16:775–785 - PubMed
-
- Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol 2006;63:1551–1555 - PubMed
-
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 - PubMed

